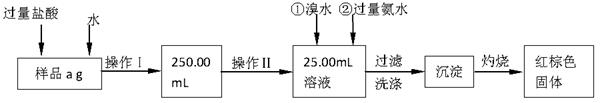
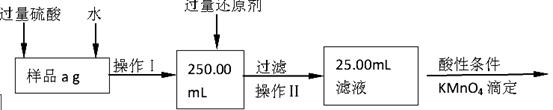
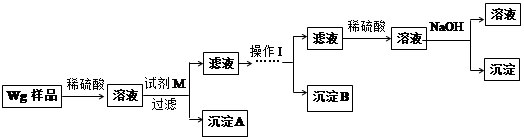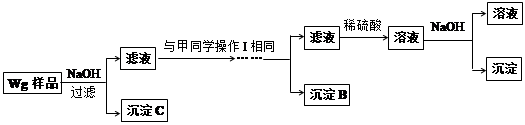��Ŀ����
�ۼ��ǹ���������ȡ����������ָ�Ƶ�һ����Ҫ�ķ�����AgNO3���ַ���������һ�֣��˵������к��գ����ֶ�����ֽ����ָ���߾�����ֽ�ϡ��������Һ��С��Ϳ��ֽ�ϣ���Һ���е����ʾ������е����ʢ����ã��������ʢۣ����ʢ��ڹ����£��ֽ���������ʻҺ�ɫ�����ŷ�Ӧ�Ľ��У����������࣬����ɫ��ɺ�ɫ��ָ���ߡ������л�ѧʽ��ʾ���������ʶ���ȷ���� (�� )
| A����AgNO3����NaBr����AgBr | B����AgNO3����NaCl����AgCl |
| C����AgCl�� ��AgNO3����NaCl | D����AgNO3����NaCl����Ag |
B
�����������������ĺ�Һ�к���NaCl,������AgNO3��Һ���ͻᷢ����Ӧ��NaCl+AgNO3=AgCl��+NaNO3;������AgCl���ȶ����ڹ�������·����ֽⷴӦ��2AgCl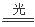 2Ag+Cl2��. �ֽ���������ʻҺ�ɫ�����ŷ�Ӧ�Ľ��У����������࣬����ɫ��ɺ�ɫ��ָ���ߡ����ѡ����B��
2Ag+Cl2��. �ֽ���������ʻҺ�ɫ�����ŷ�Ӧ�Ľ��У����������࣬����ɫ��ɺ�ɫ��ָ���ߡ����ѡ����B��
���㣺����AgNO3��Һ�ںۼ��ư��е�Ӧ�õ�֪ʶ��

���ڵؿ�����Ҫ��NaIO3����ʽ����,�ں�ˮ����Ҫ�� ����ʽ����,��������֮������ͼ��ʾ��ϵ,����ͼʾת����ϵ�Ʋ�����˵������ȷ����
����ʽ����,��������֮������ͼ��ʾ��ϵ,����ͼʾת����ϵ�Ʋ�����˵������ȷ����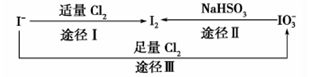
| A������KI������ֽ��ʳ����ӵ������Ƿ��е� |
B������Cl2��ʹʪ���KI������ֽ���ԭ������� |
C����ͼ��֪�����Ե�ǿ��˳��Ϊ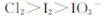 |
D��;������������1molI2,��Ӧ��ת�Ƶĵ�����Ϊ10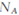 |
�����Լ��У����������ڲ���ƿ�е���
| A��Ũ���� | B������� | C��Ũ����������Һ | D����������Һ |
������Ŀ�ĵ����������������е�һ�ֶԲ������п�ʴ���Ƴɣ����������ǣ�������
| A������ | B������� | C���ռ� | D������ |
������Һ�У���ʯ��ˮ ��Na2S��Һ ��KMnO4��Һ ����ˮ ���ữ��Ba(NO3)2��Һ
��Ʒ����Һ����������SO2��CO2�������( )
| A��ֻ�Т� | B���٢� | C���٢ڢ� | D���ڢܢ� |
ij�����ڷǽ���Ԫ�ص�ԭ�Ӻ��������������Ǵ�����������һ�룬��Ԫ��( )
| A������Ȼ����ֻ�Ի���̬����ʽ���� |
| B�����ʳ������뵼����Ϻ��ά |
| C����������ﲻ���ᷴӦ |
| D����̬�⻯��ȼ����ȶ� |
����˵����ȷ����
| A�������ķ��������ݻ�����и�������ʵIJ�����еģ�Ԫ�ط����ǡ���������ǡ�ԭ�����չ����ǵȾ��Ƿ������ʵij��������� |
| B�������١���ķ����¬ɪ���������������ӵ��ȿ�ѧ����ԭ�ӽṹ����ʶ���������ش��ף�������Ҫ���õ��Ƕ����о���ʵ���о��ķ����� |
| C��ú��ʯ�͵��ۺ�������ú��������ú��Һ����ʯ�͵ķ����������仯��ʯ�͵��ѻ����ѽ⡢ú�ĸ����ǻ�ѧ�仯 |
| D��ijЩ���������γɵķ���ɸ����������״��Ѩ��ͨ���������ڷ��롢�ᴿ�����Һ���������������������ӽ���������������������� |