��Ŀ����
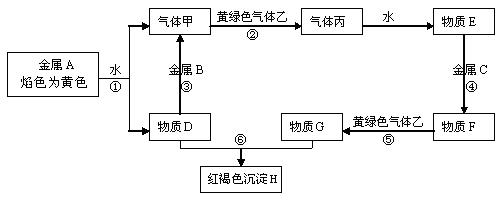
������AΪһ�ֳ�������Ԫ����һ�ֳ����ǽ���Ԫ����ɵĻ�����������������ӵĸ�����Ϊ2:3��KΪ������̬�ǽ������ʣ�J��NΪ������̬���ʣ�����Ϊ���������I��F�ڳ�����ΪҺ̬��C��DΪ�̼������壬H��ɫ��ζ���壬BΪ��ɫ��״����,LΪ�ȼҵ�еij�����Ʒ��F��Ũ��Һ��K���ȿ�����D��H��(����������δ���)
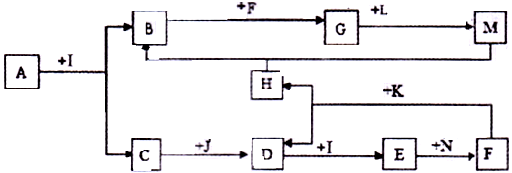
(1)H�ĽṹʽΪ: ��B�Ļ�ѧʽΪ: ��
(2)д�����б仯�Ļ�ѧ����ʽ:
A+I ��B+C�� ��
F��Ũ��Һ��K���ȿ�����D��H�� ��
(3)д�����б仯�����ӷ���ʽ:
Nͨ��E��ˮ��Һ: ��
M��ˮ��Һ��ͨ�˹�����H: ��
(4)��A��K��������������������ʵ���_ �����ڷǵ���ʵ��� (�ñ�Żش�)��

(1)H�ĽṹʽΪ: ��B�Ļ�ѧʽΪ: ��
(2)д�����б仯�Ļ�ѧ����ʽ:
A+I ��B+C�� ��
F��Ũ��Һ��K���ȿ�����D��H�� ��
(3)д�����б仯�����ӷ���ʽ:
Nͨ��E��ˮ��Һ: ��
M��ˮ��Һ��ͨ�˹�����H: ��
(4)��A��K��������������������ʵ���_ �����ڷǵ���ʵ��� (�ñ�Żش�)��
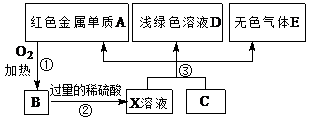
��14�֣���1��O��C��O ��1�֣���Al(OH)3��1�֣�
��2��Al2S3��6H2O��2Al(OH)3����3H2S����2�֣�
C��2H2SO4(Ũ) CO2����2SO2����2H2O��2�֣�
CO2����2SO2����2H2O��2�֣�
(3)H2SO3��Cl2��H2O��SO42����4H����2Cl����2�֣� AlO2����CO2��2H2O��Al(OH)3����HCO3����2�֣�
��4��B��C��E��I��2�֣���D��H��2�֣������һ���1�֣�ѡ��1�������÷֣�
��2��Al2S3��6H2O��2Al(OH)3����3H2S����2�֣�
C��2H2SO4(Ũ)
 CO2����2SO2����2H2O��2�֣�
CO2����2SO2����2H2O��2�֣�(3)H2SO3��Cl2��H2O��SO42����4H����2Cl����2�֣� AlO2����CO2��2H2O��Al(OH)3����HCO3����2�֣�
��4��B��C��E��I��2�֣���D��H��2�֣������һ���1�֣�ѡ��1�������÷֣�
���������BΪ��ɫ��״��������BӦ��������������������AΪһ�ֳ�������Ԫ����һ�ֳ����ǽ���Ԫ����ɵĻ�����������������ӵĸ�����Ϊ2:3�����A��һ��������Ԫ�ء���Ԫ�ص���Ҫ���ϼ��ǣ�3�ۣ���A������һ��Ԫ�صĻ��ϼ�Ӧ���ǣ�2�ۣ���Ϊ�ڢ�A��Ԫ�ء�I��F�ڳ�����ΪҺ̬��������һ����ˮ����˵��AӦ����Al2S3��������Al2O3����ΪAl2O3������ˮ��Al2S3����ˮ��������������H2S����C��H2S��I��ˮ��J��NΪ������̬���ʣ�C�ܺ�J��Ӧ�����J��������DΪ�̼������壬��D��SO2��SO2����ˮ���������ᡣKΪ������̬�ǽ������ʣ�F��Ũ��Һ��K���ȿ�����D��H��H��ɫ��ζ���壬����HӦ����CO2��K��̼��F�����ᣬ���N���������������������ᷴӦ������������LΪ�ȼҵ�еij�����Ʒ��L�ܺ���������Ӧ������L���������ƣ�M��ƫ�����ơ�
��1���������Ϸ�����֪��H�ĽṹʽΪO��C��O��B�Ļ�ѧʽΪAl(OH)3��
��2���������Ϸ�����֪��A+I ��B+C �Ļ�ѧ����ʽΪAl2S3��6H2O��2Al(OH)3����3H2S����F��Ũ��Һ��K���ȿ�����D��H�Ļ�ѧ����ʽΪC��2H2SO4(Ũ)
 CO2����2SO2����2H2O��
CO2����2SO2����2H2O��(3)�������Ϸ�����֪�� Nͨ��E��ˮ��Һ�����ӷ���ʽΪH2SO3��Cl2��H2O��SO42����4H����2Cl����M��ˮ��Һ��ͨ�˹�����H�����ӷ���ʽΪAlO2����CO2��2H2O��Al(OH)3����HCO3����
��4�����ڵ���ƽ��ĵ������������ʣ������A��K��������������������ʵ�������������H2S�������ᡢˮ������ѡB��C��E��I������ˮ��������״̬�²��ܵ���Ļ�����Ƿǵ���ʣ��������ڷǵ���ʵ���CO2��SO2����ѡD��H��

��ϰ��ϵ�д�
�����Ŀ