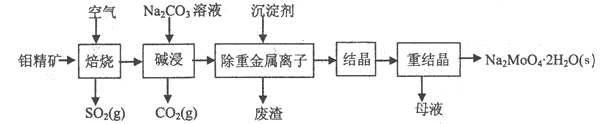
��Ŀ����
����Ŀ��ʵ��������450 mL 0.5 mol��L-1��NaCl��Һ�������²������裺
�ٰѳ�����NaCl�������С�ձ��У�����������ˮ�ܽ⡣
�ڰѢ�������ҺС��ת����ѡ����ƿ�С�
�ۼ���������ƿ�м�����ˮ��Һ���̶�1��2 cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ��ײ���̶������С�
������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ��������ҡ�ȡ�
�ݽ�����ƿ�����������ҡ�ȡ�
����д���пհף�
��1�������������ȷ˳��Ϊ______________(�����)��
��2����ʵ���õ���������������ƽ��ҩ�ס����������ձ���__________________��
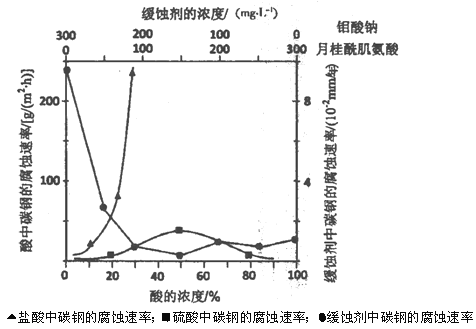
��3��ijͬѧ�۲�Һ��������ͼ��ʾ����������ҺŨ�Ƚ��к�Ӱ�죿__________(����ƫ������ƫ����������Ӱ����)��

��4�������������������������ҺŨ�Ƚ��к�Ӱ�죿������ˮʱ���������˿̶�__________��(����ƫ������ƫ����������Ӱ����)
��5����ʹ��5 mol��L-1��NaCl��Һϡ��������450 mL 0.5 mol��L-1��NaCl��Һ������Ҫ5 mol��L-1��NaCl��Һ________mL��
���𰸡��٢ڢܢۢ�500ml����ƿ ��ͷ�ι� ����Ͳ��ƫ��ƫ��50.0 ��50��
��������
��1����������һ�����ʵ���Ũ����Һ�IJ����������
��2���������Ʋ���ѡ��ʹ��������Ȼ���жϻ�ȱ�ٵ�������
��3��(4)�������������ʵ����ʵ�������Һ�����Ӱ�죬����C=n/V������������
��5������ϡ�Ͷ��ɼ���
��1������ 500mL 0.5mol��L-1 ��NaCl��Һ�IJ�������Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���ȷ�IJ���˳��Ϊ���٢ڢܢۢݣ�
��2������ 500mL 0.5mol��L-1 ��NaCl��Һ�IJ�������Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ��õ�������Ϊ����ƽ��ҩ�ס���Ͳ���ձ��� 500mL ����ƿ������������ͷ�ιܣ����Ի�ȱ�ٵ�����Ϊ�� 500mL ����ƿ����ͷ�ι� ����Ͳ�� ��
��3��ijͬѧ�۲�Һ����������ͼ�����ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ�
��4��������ˮʱ���������˿̶ȣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
��5����ʹ��5 mol��L-1��NaCl��Һϡ��������450 mL 0.5 mol��L-1��NaCl��Һ������Ҫ5 mol��L-1��NaCl��Һ=![]() =50.0mL��
=50.0mL��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�����Ŀ����������ĵ���ƽ�ⳣ�����±���
���� | HCOOH | HClO |
|
|
����ƽ�ⳣ�� |
|
|
|
|
��1��������![]()
![]()
![]()
![]()
![]() ��Һ��pH�ɴ�С�Ĺ�ϵΪ______��
��Һ��pH�ɴ�С�Ĺ�ϵΪ______��
��2��Ũ�Ⱦ�Ϊ![]() ��
��![]() ��
��![]() �Ļ����Һ�У�
�Ļ����Һ�У� ![]() ��
��![]() ��
��![]() ��
��![]() Ũ�ȴӴ�С��˳��Ϊ______��
Ũ�ȴӴ�С��˳��Ϊ______��
��3������![]() ͨ�뵽������NaClO��Һ�з�����Ӧ�����ӷ���ʽ______��
ͨ�뵽������NaClO��Һ�з�����Ӧ�����ӷ���ʽ______��
��4�������£� ![]() ��HCOOH��Һ��
��HCOOH��Һ��![]() ��NaOH��Һ�������Ϻ���Һ������Ũ���ɴ�С��˳��Ϊ______��
��NaOH��Һ�������Ϻ���Һ������Ũ���ɴ�С��˳��Ϊ______��