��Ŀ����
��15�֣����ŵ���������Ⱦ���������أ����ҽ��ڡ�ʮ���塱�ڼ�Ӵ�Ե��������ŷŵĿ������ȡ�Ŀǰ����������������Ⱦ�ж��ַ�����
(1)�û���̿��ԭ��������������йط�ӦΪ��C(s)��2NO(g) N2(g)��CO2 (g) ��H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)��CO2 (g) ��H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K�� ��������λС������
��30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ��
����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3����÷�Ӧ�ġ�H 0����������� ������������
(2)��CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H����574 kJ��mol��1
��CH4(g)��4NO(g)��2N2(g)��CO2(g)��2H2O(g) ��H����1160 kJ��mol��1
��H2O(g)��H2O(l) ��H����44.0 kJ��mol��1
д��CH4(g)��NO2(g)��Ӧ����N2(g)��CO2(g)��H2O(1)���Ȼ�ѧ����ʽ ��
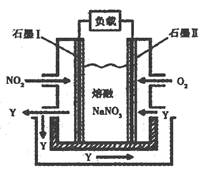
(3)��NO2��O2������NaNO3��ɵ�ȼ�ϵ��װ����ͼ��ʾ����ʹ�ù�����ʯīI�缫��Ӧ����һ��������Y���йص缫��Ӧ�ɱ�ʾΪ ��
(1)�û���̿��ԭ��������������йط�ӦΪ��C(s)��2NO(g)
 N2(g)��CO2 (g) ��H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)��CO2 (g) ��H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�| Ũ��/mol��L��1 ʱ��/min | NO | N2 | CO2 |
| 0 | 0.100 | 0 | 0 |
| 10 | 0.058 | 0.021 | 0.021 |
| 20 | 0.040 | 0.030 | 0.030 |
| 30 | 0.040 | 0.030 | 0.030 |
| 40 | 0.032 | 0.034 | 0.017 |
| 50 | 0.032 | 0.034 | 0.017 |
��30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ��
����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3����÷�Ӧ�ġ�H 0����������� ������������
(2)��CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H����574 kJ��mol��1
��CH4(g)��4NO(g)��2N2(g)��CO2(g)��2H2O(g) ��H����1160 kJ��mol��1
��H2O(g)��H2O(l) ��H����44.0 kJ��mol��1
д��CH4(g)��NO2(g)��Ӧ����N2(g)��CO2(g)��H2O(1)���Ȼ�ѧ����ʽ ��
(3)��NO2��O2������NaNO3��ɵ�ȼ�ϵ��װ����ͼ��ʾ����ʹ�ù�����ʯīI�缫��Ӧ����һ��������Y���йص缫��Ӧ�ɱ�ʾΪ ��

(1)��0.56 �ڼ�СCO2��Ũ�ȣ������𰸾��ɣ� �ۣ�
(2)CH4(g)��2NO2(g)��N2(g)��CO2(g)��2H2O(l) ��H����955 kJ��mol��1
(3)NO2��NO3����e����N2O5
(2)CH4(g)��2NO2(g)��N2(g)��CO2(g)��2H2O(l) ��H����955 kJ��mol��1
(3)NO2��NO3����e����N2O5
��1���ٸ��ݱ������ݿ�֪����Ӧ���е�20min���������ʵ�Ũ�Ȳ��ٷ����仯������ƽ�ⳣ����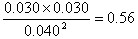
��30min��4minʱNO��CO2Ũ�ȼ�С��������Ũ���������Ըı�������Ǽ�СCO2��Ũ�ȡ�
��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3��˵�������¶ȷ�Ӧ���Ũ��������˷�Ӧ���淴Ӧ�����ƶ���������Ӧ�Ƿ��ȷ�Ӧ��
��2�������˹���ɵ�Ӧ�ã����٣��ڣ���2���ۡ�2���õ�CH4(g)��2NO2(g)��N2(g)��CO2(g)��2H2O(l)�����Է�Ӧ���ǣ���574 kJ��mol��1��1160 kJ��mol��1����2��44.0 kJ��mol��1��2����955 kJ��mol��1��
��3������װ��ͼ��֪��Y����ʯīI���ɵģ���ʯīIͨ�����NO2�������Ǹ�����ʧȥ���ӣ���˷�ӦʽΪNO2��NO3����e����N2O5��
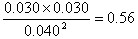
��30min��4minʱNO��CO2Ũ�ȼ�С��������Ũ���������Ըı�������Ǽ�СCO2��Ũ�ȡ�
��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3��˵�������¶ȷ�Ӧ���Ũ��������˷�Ӧ���淴Ӧ�����ƶ���������Ӧ�Ƿ��ȷ�Ӧ��
��2�������˹���ɵ�Ӧ�ã����٣��ڣ���2���ۡ�2���õ�CH4(g)��2NO2(g)��N2(g)��CO2(g)��2H2O(l)�����Է�Ӧ���ǣ���574 kJ��mol��1��1160 kJ��mol��1����2��44.0 kJ��mol��1��2����955 kJ��mol��1��
��3������װ��ͼ��֪��Y����ʯīI���ɵģ���ʯīIͨ�����NO2�������Ǹ�����ʧȥ���ӣ���˷�ӦʽΪNO2��NO3����e����N2O5��

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
�����Ŀ

 ��
��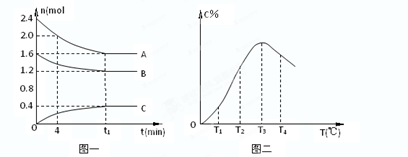
 2C(g)����2s ����C��Ũ��Ϊ0.6mol?L-1�������м���˵����������ȷ���� ( )
2C(g)����2s ����C��Ũ��Ϊ0.6mol?L-1�������м���˵����������ȷ���� ( ) 2C(g)
2C(g) 
 nZ��g��+2W��g�� , 5 minĩ��Ӧ�ﵽƽ��״̬����ʱ������0.2molW������֪��ZŨ�ȱ仯����ʾ�ķ�Ӧƽ������Ϊ0.01mol/L��min
nZ��g��+2W��g�� , 5 minĩ��Ӧ�ﵽƽ��״̬����ʱ������0.2molW������֪��ZŨ�ȱ仯����ʾ�ķ�Ӧƽ������Ϊ0.01mol/L��min MgCl2��6H2O
MgCl2��6H2O
 2YX3(g) ��H ��Ӧ�����ʵ���Ũ����ʱ��仯���£�
2YX3(g) ��H ��Ӧ�����ʵ���Ũ����ʱ��仯���£�