��Ŀ����
(11��)��֪����������Ԫ��X��Y��Z��W��ԭ����������������X��Y��ԭ������֮�͵���Z��ԭ��������X��Z���γ�X2Z��X2Z2���ֻ����W�Ƕ���������Ԫ���а뾶����Ԫ�ء�
�� W�����ڱ��е�λ�ã� ��
����һ�������£��ݻ�Ϊ1L�ܱ������м���1.2molX2��0.4molY2���������·�Ӧ��
3X2 (g) + Y2(g) 2YX3(g) ��H ��Ӧ�����ʵ���Ũ����ʱ��仯���£�
2YX3(g) ��H ��Ӧ�����ʵ���Ũ����ʱ��仯���£�

�ٴ˷�Ӧ��ƽ�ⳣ������ʽΪ (�û�ѧʽ��ʾ) �� K= ��
���������¶�ƽ�ⳣ��K��С�����H 0(�����)��
��A1������Ԫ��������Ԫ����ɵĵ���ʣ���Һ�ʼ��ԣ���0.1mol��L-1��A1��Һϡ����ԭ�����10������Һ��pH=12����A1�ĵ���ʽΪ ��
��B1��B2��������Ԫ�������γɵ�ǿ����ʣ�����Һ�����ԣ���ͬŨ��ʱB1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȣ���ԭ���� ��
��A2��B1��Ӧ����B2����0.2mol/LA2��0.1mol/L B1�������Ϻ���Һ������Ũ�ȴ�С��ϵΪ ��
�� W�����ڱ��е�λ�ã� ��
����һ�������£��ݻ�Ϊ1L�ܱ������м���1.2molX2��0.4molY2���������·�Ӧ��
3X2 (g) + Y2(g)
 2YX3(g) ��H ��Ӧ�����ʵ���Ũ����ʱ��仯���£�
2YX3(g) ��H ��Ӧ�����ʵ���Ũ����ʱ��仯���£�
�ٴ˷�Ӧ��ƽ�ⳣ������ʽΪ (�û�ѧʽ��ʾ) �� K= ��
���������¶�ƽ�ⳣ��K��С�����H 0(�����)��
��A1������Ԫ��������Ԫ����ɵĵ���ʣ���Һ�ʼ��ԣ���0.1mol��L-1��A1��Һϡ����ԭ�����10������Һ��pH=12����A1�ĵ���ʽΪ ��
��B1��B2��������Ԫ�������γɵ�ǿ����ʣ�����Һ�����ԣ���ͬŨ��ʱB1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȣ���ԭ���� ��
��A2��B1��Ӧ����B2����0.2mol/LA2��0.1mol/L B1�������Ϻ���Һ������Ũ�ȴ�С��ϵΪ ��
(1)��������,IA(2��)
(2)��K= C(NH3)2/[C(H2)3C(N2)] (1��) 100/27 (mol/L)-2 (2��) �ڣ�(1��)
(3)NaOH����ʽ(1��)����
(4)��������������������ˮ�ĵ��룬笠����ӵĴ��ڴٽ���ˮ�ĵ���
(2��)
(5)C(NH4+)�� C(NO3-)��C(OH-) ��C(H+)(2��)
(2)��K= C(NH3)2/[C(H2)3C(N2)] (1��) 100/27 (mol/L)-2 (2��) �ڣ�(1��)
(3)NaOH����ʽ(1��)����
(4)��������������������ˮ�ĵ��룬笠����ӵĴ��ڴٽ���ˮ�ĵ���
(2��)
(5)C(NH4+)�� C(NO3-)��C(OH-) ��C(H+)(2��)
����Ԫ��ԭ�ӽṹ�ص���Ƴ���Ԫ��ΪXΪ�⣬YΪ����ZΪ����WΪ
�ơ��Ӷ�ͨ������˼�����н��
�ơ��Ӷ�ͨ������˼�����н��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 N2(g)��CO2 (g) ��H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)��CO2 (g) ��H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�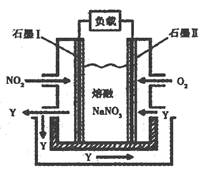

 Cr2O72������ɫ��+H2O
Cr2O72������ɫ��+H2O 2c(g)����H��0���ﵽƽ��ı�һ������(X)��������(Y)�ı仯һ������ͼ�����ߵ���
2c(g)����H��0���ﵽƽ��ı�һ������(X)��������(Y)�ı仯һ������ͼ�����ߵ���
 2Z(g) + R(g)����Ӧƽ��ʱ����֪X ��Y ��ת���ʷֱ�Ϊ30%��60%��
2Z(g) + R(g)����Ӧƽ��ʱ����֪X ��Y ��ת���ʷֱ�Ϊ30%��60%��  CH3OH(g) + H2O(g) ��H=" -49.0" kJ��mol-1
CH3OH(g) + H2O(g) ��H=" -49.0" kJ��mol-1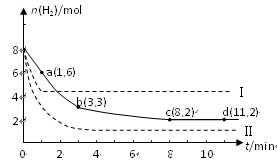

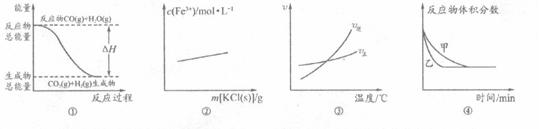
 CO2��g����H2��g�����еġ�H����0
CO2��g����H2��g�����еġ�H����0 ��ѹǿ��
��ѹǿ��