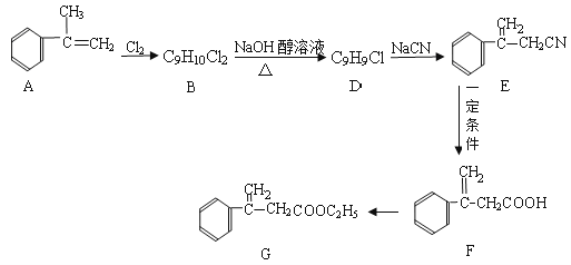
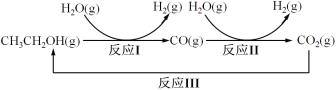
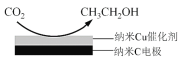
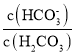��Ŀ����
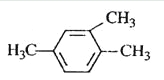
����Ŀ����ͼ��A��B��C��D��ͬ���ڻ�ͬ���������Ԫ�أ�
D | ||
A | B | C |
(1)��֪��AԪ�ص���ͼ�Ϊ-3�ۣ�������������ﺬ��56.34%��ԭ�Ӻ�������������������1������AԪ��ԭ�ӵ�������Ϊ_____��
(2)д��Ԫ�ط��ţ�A_____��C_____��D____��
(3)A��B��C����Ԫ������������ˮ������������ǿ����____(�ѧʽ)��
(4)B��D����Ԫ�غ�����ɵ���̬�⻯�����ȶ�����ǿ����___����ԭ����ǿ��____(�ѧʽ)��
���𰸡�31 P Cl O HClO4 H2O H2S
��������
A��B��C��D��ͬ���ڻ�ͬ���������Ԫ�أ�AԪ����ͼ�Ϊ-3�ۣ��������Ϊ+5�ۣ��������������ΪA2O5������56.34%������A�����ԭ������Ϊa����![]() ��100%=56.34%�����a=31����A��������Ϊ31������A��ԭ�Ӻ�������������������1������������Ϊ
��100%=56.34%�����a=31����A��������Ϊ31������A��ԭ�Ӻ�������������������1������������Ϊ![]() =15����AΪP�������λ�ù�ϵ��֪BΪS��CΪCl��DΪO��
=15����AΪP�������λ�ù�ϵ��֪BΪS��CΪCl��DΪO��
(1)�ɷ�����AΪP��AԪ��ԭ�ӵ�������Ϊ31���ʴ�Ϊ��31��
(2)Ԫ�ط��ţ�AΪP��CΪCl��DΪO���ʴ�Ϊ��P��Cl��O��
(3)P��S��Cl����Ԫ������������ˮ��������ΪH3PO4��H2SO4��HClO4���ǽ�����Cl>S>P����������ǿ����HClO4���ʴ�Ϊ��HClO4��
(4)S��O����Ԫ�غ�����ɵ���̬�⻯��ֱ���H2S��H2O���ǽ�����O>S����H2O���ȶ��Խϴ�H2S�Ļ�ԭ�Խ�ǿ���ʴ�Ϊ��H2O��H2S��

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�