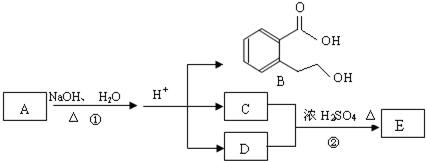
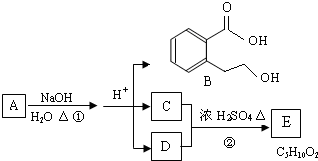
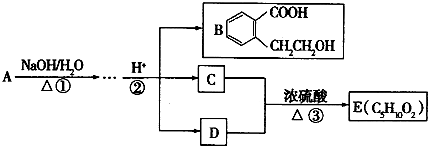
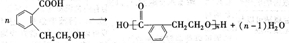��Ŀ����
��������������Ӧ�Ļ�ѧ����ʽ���ж��й����ʵĻ�ԭ��ǿ��˳�� ��
��I2+SO2+2H2O=H2SO4+2HI ��2FeCl2+Cl2=2FeCl3 ��FeCl3+2HI=2FeCl2+2HCl+I2
A��I�D��Fe2+��Cl�D��SO2 B��Cl�D��Fe2+��SO2��I�D
C��Fe2+��I�D��Cl�D��SO2 D��SO2��I�D��Fe2+��Cl�D
D

��ϰ��ϵ�д�
�����Ŀ
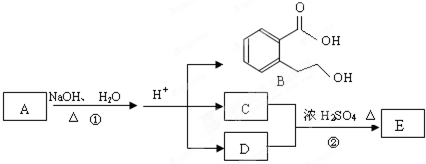
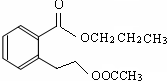
 ����1��
����1��