��Ŀ����
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ��C��D����Է���������ȣ���EΪ��֧���Ļ����
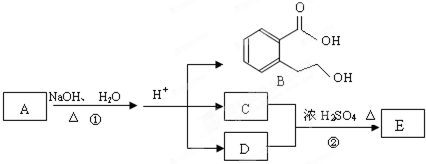
������ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ������E�ķ���ʽΪ
��2��������B���ܷ����ķ�Ӧ��
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ d��������Ӧ e��ˮ�ⷴӦ f�� �û���Ӧ
��3����Ӧ�ڵĻ�ѧ����ʽ��
��4��C�����еĹ�����������
 ��
��
��5��ͬʱ������������������B��ͬ���칹�����Ŀ��
���м��ȡ�������ṹ�����ڷǷ����������� FeCl3 ��Һ������ɫ��Ӧ��
д����������һ��ͬ���칹��Ľṹ��ʽ ����1��
����1�� ����1����
����1����

������ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ������E�ķ���ʽΪ
C5H10O2
C5H10O2
����2��������B���ܷ����ķ�Ӧ��
e
e
������ĸ��ţ���a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ d��������Ӧ e��ˮ�ⷴӦ f�� �û���Ӧ
��3����Ӧ�ڵĻ�ѧ����ʽ��
CH3COOH+CH3CH2CH2OH
CH3COOCH2CH2CH3+H2O
| Ũ���� |
| �� |
CH3COOH+CH3CH2CH2OH
CH3COOCH2CH2CH3+H2O
��| Ũ���� |
| �� |
��4��C�����еĹ�����������
�Ȼ�
�Ȼ�
��A�Ľṹ��ʽ��

��5��ͬʱ������������������B��ͬ���칹�����Ŀ��
4
4
�������м��ȡ�������ṹ�����ڷǷ����������� FeCl3 ��Һ������ɫ��Ӧ��
д����������һ��ͬ���칹��Ľṹ��ʽ
 ����1��
����1�� ����1��
����1��������E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%����̼ԭ����ĿΪ
=5��Hԭ����ĿΪ
=10����ԭ����Ŀ=
=2����E�ķ���ʽΪC5H10O2��E��C��D��Ӧ���ɣ�C�ܺ�̼�����Ʒ�Ӧ����CΪ���ᣬDΪ�������߹���5��Cԭ�ӣ�����C��D����Է���������ȣ���CΪ���ᡢDΪ������E��֧����DӦΪ1-������EΪCH3COOCH��CH3��2��A����B�����ᡢ��������������Ӧ�����ɵIJ����ṹ��ʽΪ�� ��
��
| 102��58.8% |
| 12 |
| 102��9.8% |
| 1 |
| 102-12��5-10 |
| 16 |
 ��
������⣺E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%����̼ԭ����ĿΪ
=5��Hԭ����ĿΪ
=10����ԭ����Ŀ=
=2����E�ķ���ʽΪC5H10O2��E��C��D��Ӧ���ɣ�C�ܺ�̼�����Ʒ�Ӧ����CΪ���ᣬDΪ�������߹���5��Cԭ�ӣ�����C��D����Է���������ȣ���CΪ���ᡢDΪ������E��֧����DӦΪ1-������EΪCH3COOCH��CH3��2��A����B�����ᡢ��������������Ӧ�����ɵIJ����ṹ��ʽΪ�� ��
��
��1��ͨ�����Ϸ���֪��E�ķ���ʽΪ��C5H10O2��
�ʴ�Ϊ��C5H10O2��
��2��B�к����Ȼ������ǻ��ͱ����������Ӵ��ǻ�̼ԭ�����ڵ�̼ԭ�����������ӣ�����B�ܷ����ӳɡ�ȡ������������ȥ���û��ȷ�Ӧ�����ܷ���ˮ�ⷴӦ��
�ʴ�Ϊ��e��
��3����Ϊ����ͱ�����������Ӧ����Ӧ����ʽΪ��CH3COOH+CH3CH2CH2OH
CH3COOCH2CH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2CH2OH
CH3COOCH2CH2CH3+H2O��
��4��������������֪��C�����ᣬ����C�����Ȼ���AΪ ��
��
�ʴ�Ϊ���Ȼ��� ��
��
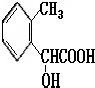
��5������������B��ͬ���칹���У���������ȡ����������һ�����ǻ��������������ᡢ��������ֱ����Ӧ�ķ��㴼���������Ӧ���ɵ��������ʣ�����������ͬ���칹��ṹΪ�� ���ʷ��������Ĺ���4�֣�
���ʷ��������Ĺ���4�֣�
�ʴ�Ϊ��4�� ����1�֣�
����1�֣�
| 102��58.8% |
| 12 |
| 102��9.8% |
| 1 |
| 102-12��5-10 |
| 16 |
 ��
����1��ͨ�����Ϸ���֪��E�ķ���ʽΪ��C5H10O2��
�ʴ�Ϊ��C5H10O2��
��2��B�к����Ȼ������ǻ��ͱ����������Ӵ��ǻ�̼ԭ�����ڵ�̼ԭ�����������ӣ�����B�ܷ����ӳɡ�ȡ������������ȥ���û��ȷ�Ӧ�����ܷ���ˮ�ⷴӦ��
�ʴ�Ϊ��e��
��3����Ϊ����ͱ�����������Ӧ����Ӧ����ʽΪ��CH3COOH+CH3CH2CH2OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3COOH+CH3CH2CH2OH
| Ũ���� |
| �� |
��4��������������֪��C�����ᣬ����C�����Ȼ���AΪ
 ��
���ʴ�Ϊ���Ȼ���
 ��
����5������������B��ͬ���칹���У���������ȡ����������һ�����ǻ��������������ᡢ��������ֱ����Ӧ�ķ��㴼���������Ӧ���ɵ��������ʣ�����������ͬ���칹��ṹΪ��
 ���ʷ��������Ĺ���4�֣�
���ʷ��������Ĺ���4�֣��ʴ�Ϊ��4��
 ����1�֣�
����1�֣����������⿼���л���ĺϳ����ƶϣ������������ˮ���֪ʶ����Ϸ�Ӧ������ת���ж���ṹ�ǽ���Ĺؼ�������������Ŀʱע���ھ���Ŀ�еĹؼ���Ϣ���������ƻ����Ƶķ�ʽ��һͻ�ƣ��ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ




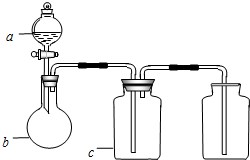 ��ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ��������ǣ�������
��ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ��������ǣ�������


 CH2-CH2
CH2-CH2 n��CH2=CH2+H2O
n��CH2=CH2+H2O