��Ŀ����
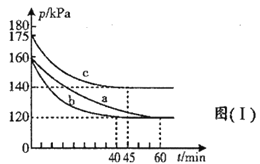
����Ŀ��A����һ��������Mg��Al�����Ͷ��400 mLϡ�����У�����ȫ���ܽⲢ�������塣����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ��
���㣺
��1��Mg��Al��������Ϊ________g��
��2����������ʵ���Ũ��Ϊ________��
��3������H2�����ʵ���Ϊ________��
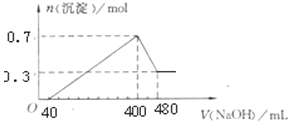
B����һ���������ۡ�������������þ�Ļ������뵽300mL ,4mol/L��ϡ�����У����ɱ�״����6.72L���塣��Ӧ�����Һ�еμ�һ�����ʵ���Ũ�ȵ�KOH��Һʱ�����ó��������ʵ�����mol����μ�KOH��Һ�������mL��֮��Ĺ�ϵ��ͼ��ʾ��
��1��OA��Ӧ��Ӧ�����ӷ���ʽΪ_________��BC��Ӧ��Ӧ�����ӷ���ʽΪ____________��
��2��c��KOH��=_________mol/L��������������������ʵ���Ϊ_____mol��
��3��A���Ӧ����ֵΪ_______��
��4������ѧ�Σ�ʵ���������B���Ӧ����Һ�е�������ʱ�����۲쵽��������___________��
��5����C���Ӧ����Һ��ͨ��������CO2��������Ӧ�����ӷ���ʽΪ_____________��
���𰸡�18 g 2.5 mol/l 0.9mol H+ +OH-=H2O Al(OH)3 +OH-=AlO2-+2H2O 4 0.1 200 ����ɫ�ܲ����۲죬�������ɫ CO2 +AlO2- +2H2O=Al(OH)3��+HCO3-
��������
A. ��ͼ���֪���ӿ�ʼ������NaOH��Һ40 mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H2SO4+2NaOH=Na2SO4+2H2O����V(NaOH) = 400 mLʱ�����������ʱΪMg(OH)2��Al(OH)3���������ʵ���֮��Ϊ0.7 mol����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn(Na2SO4)����400 mL����������Һ�к��е�n(NaOH)��0.5������400mL��ʼ��NaOH�ܽ�Al(OH)3��������ӦNaOH+Al(OH)3 = NaAlO2+2H2O�����������ټ���ʱֻ��Mg(OH)2�����ʵ���Ϊ0.3 mol�����Գ��������ʱ��Mg(OH)2Ϊ0.3 mol��Al(OH)3Ϊ0.7 mol-0.3 mol=0.4 mol�����Ըý�����n(NaOH) = n[Al(OH)3] = 0.4 mol���������Ƶ�Ũ��Ϊ![]() = 5 mol/L��
= 5 mol/L��
��1����Ԫ���غ��֪n(Al) = n[Al(OH)3]��n(Mg) = n[Mg(OH)2]���ڸ���m =n M������Ե����������������������������
��2�����������ʱΪMg(OH)2��Al(OH)3����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn(NaOH)= 2 n(Na2SO4)������������غ�n(H2SO4) = n(Na2SO4)���ٸ���c=![]() �����㣻
�����㣻
��3�����ݵ���ת���غ��֪2n(H2) = 3n(Al) + 2n(Mg)���ݴ˼���n(H2)��
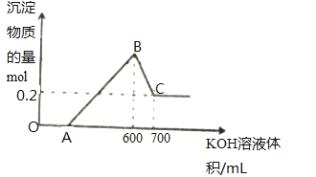
B. ��ͼ��֪���ӿ�ʼ������KOH��Һ��A��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H2SO4+2KOH=K2SO4+2H2O�������μ�KOH��Һ��������������ҺΪ600 mLʱ�����������ʱΪMg(OH)2��Al(OH)3����ҺΪ�������Һ���ټ����μ�KOH��Һ�������������������ط�Ӧ����ƫ�������ˮ��������ӦAl(OH)3+KOH=KAlO2+2H2O���ʷ�Ӧ�����Һ����ΪKAlO2��ͨ��������CO2�������������������������Ҳ��ܽ⣬�ݴ˽��н���ڽ�����ɫ��Ӧʱ��Ϊ���������ӶԼ����ӵĸ��ţ���Ҫ����ɫ�ܲ����۲졣
A. ��ͼ���֪���ӿ�ʼ������NaOH��Һ40mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H2SO4+2NaOH = Na2SO4+2H2O����V(NaOH) = 400mLʱ�����������ʱΪMg(OH)2��Al(OH)3���������ʵ���֮��Ϊ0.7 mol����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn(Na2SO4)����400 mL����������Һ�к��е�n(NaOH)��0.5������400 mL��ʼ��NaOH�ܽ�Al(OH)3��������ӦNaOH+Al(OH)3=NaAlO2+2H2O�����������ټ��٣���ʱȫ��ΪMg(OH)2�����ʵ���Ϊ0.3 mol�����Գ��������ʱ��Mg(OH)2Ϊ0.3 mol��Al(OH)3Ϊ0.7 mol-0.3 mol=0.4mol�����Ըý�����n(NaOH) = n[Al(OH)3] = 0.4mol���������Ƶ�Ũ��Ϊ![]() = 5 mol/L��
= 5 mol/L��
��1����Ԫ���غ��֪n(Al) = n[Al(OH)3] = 0.4 mol��n(Mg) = n[Mg(OH)2] = 0.3 mol����Mg��Al��������Ϊ0.4mol��27g/mol+0.3mol��24g/mol = 18 g���ʴ�Ϊ��18 g��
��2�����������ʱΪMg(OH)2��Al(OH)3����Һ������ΪNa2SO4��������Ԫ���غ��֪��ʱn(NaOH)=2n(Na2SO4)=0.4L��5mol/L = 2 mol��������n(Na2SO4) = 1 mol�����������Ũ��Ϊc = ![]() = 2.5 mol/L���ʴ�Ϊ��2.5 mol/L��
= 2.5 mol/L���ʴ�Ϊ��2.5 mol/L��
��3���ɣ�1���п�֪n(Al) = 0.4 mol��n(Mg) = 0.3 mol�����ݵ���ת���غ��֪2n(H2) = 3n(Al)+2n(Mg) = 3��0.4mol+2��0.3mol=1.8 mol������n(H2) = 0.9 mol���ʴ�Ϊ��0.9 ol��
B. ��ͼ��֪���ӿ�ʼ������KOH��Һ��A��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H2SO4+2KOH=K2SO4+2H2O�������μ�KOH��Һ��������������ҺΪ600 mLʱ�����������ʱΪMg(OH)2��Al(OH)3��������Ӧ��3OH+Al3+=Al(OH)3����2OH+Mg2+=Mg(OH)2������ҺΪ�������Һ���ټ����μ�KOH��Һ�������������������ط�Ӧ����ƫ�������ˮ��������ӦAl(OH)3+KOH=KAlO2+2H2O�����������ܽ⡣
��1��OA�Σ��������������ᷴӦ��������غ�ˮ�����ӷ���ʽ��H++OH=H2O��BC�Σ������μ�KOH��Һ�������������������ط�Ӧ����ƫ�������ˮ��������ӦAl(OH)3+KOH=KAlO2+2H2O�����ӷ���ʽ��Al(OH)3 +OH-=AlO2-+2H2O���ʴ�Ϊ��H++OH=H2O��Al(OH)3 +OH-=AlO2-+2H2O��
��2����������ʵ���Ϊ��0.3 L��4 mol/L = 1.2 mol��B����ҺΪ�������Һ������2K+SO42������������ʵ���Ϊ2.4 mol�������������������ʵ���Ϊ2.4 mol���������������ʵ���Ũ��Ϊ��2.4 mol��0.6 L=4 mol/L���������뵽300 mL4 mol/L��ϡ�����У����ɱ�״����6.72 L���壬���ʵ���Ϊ![]() =0.3 mol����2Al3H2������֪�������ʵ���Ϊ0.2 mol��BC�����������������ʵ���Ϊ��0.1 L��4 mol/L=0.4 mol��������Ӧ��Al(OH)3 +OH-=AlO2-+2H2O����֪�����������ʵ���Ϊ0.4 mol��������ԭ�Ӹ����غ㣬���������������ʵ���Ϊ��0.4 mol 0.3 mol =0.1 mol���ʴ�Ϊ��4��0.1��
=0.3 mol����2Al3H2������֪�������ʵ���Ϊ0.2 mol��BC�����������������ʵ���Ϊ��0.1 L��4 mol/L=0.4 mol��������Ӧ��Al(OH)3 +OH-=AlO2-+2H2O����֪�����������ʵ���Ϊ0.4 mol��������ԭ�Ӹ����غ㣬���������������ʵ���Ϊ��0.4 mol 0.3 mol =0.1 mol���ʴ�Ϊ��4��0.1��
��3�����ݼ���700 mL�������غ�������ʵ���Ϊ0.2 mol������Һ�к�þ�������ʵ���Ϊ0.2 mol������þ��������0.4 mol�������أ�����0.4 mol��������Ҫ���������������ʵ���Ϊ0.4mol��3 = 1.2 mol������OA�����������������ʵ���Ϊ��0.6 L��4 mol/L0.4 mol1.2 mol = 0.8 mol�������������������![]() = 0.2 L����200 mL���ʴ�Ϊ��200��
= 0.2 L����200 mL���ʴ�Ϊ��200��
��4����������ӿ�������ɫ��Ӧ������ɫ�ܲ����۲죬�������ɫ���ʴ�Ϊ������ɫ�ܲ����۲죬�������ɫ��
��5��C����ҺΪƫ�������Һ��������̼����ˮ�γ�����̼�ᣬ��ͨ������������̼��Ӧ������������������̼����أ���Ӧ�����ӷ���ʽ��CO2 +AlO2- +2H2O=Al(OH)3��+HCO3-���ʴ�Ϊ��CO2 +AlO2- +2H2O=Al(OH)3��+HCO3-��