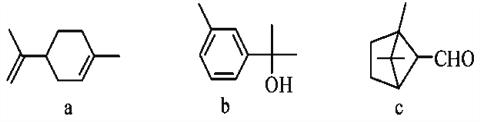
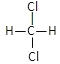
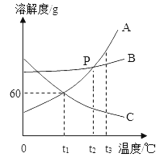
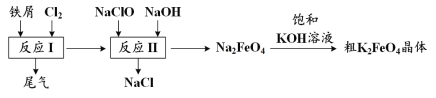��Ŀ����
����Ŀ��ijС��ͬѧΪ̽��H2O2��H2SO3��Br2��������ǿ�����������ʵ��(�г���������ȥ��װ�õ��������Ѽ���)��
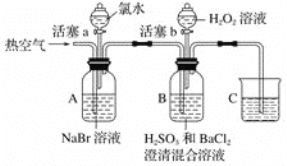
ʵ���¼���£�
ʵ����� | ʵ������ | |
�� | ����a���μ���ˮ���رջ���a | A����Һ��Ϊ����ɫ |
�� | �����ȿ��� | A�к���ɫ���Ա�dz��B�������ݣ�����������ɫ���������Һ��ɫ�����Ա仯 |
�� | ֹͣ�������������b����μ���H2O2��Һ | ��ʼʱ��ɫ�����Ա仯�������μ�H2O2��Һ��һ��ʱ����Һ��ɺ���ɫ |
��ش��������⣺
��1��A�з�Ӧ�����ӷ���ʽ��______��
��2��ʵ����������ȿ�����Ŀ����_____��
��3��װ��C��������_____��C��ʢ�ŵ�ҩƷ��_____��
��4��ʵ��������Һ��ɺ���ɫ�����Ӧ�����ӷ���ʽ��_____��
��5��������ʵ��ó��Ľ�����______��
��6��ʵ�鷴˼��
����ͬѧ��Ϊʵ�����������ȿ���������ţ�5���н��۵ĵó�������Ϊ�Ƿ���ţ�������________��
��ʵ�������ʼʱ��ɫ�����Ա仯��ԭ����(д��һ������)______��
���𰸡�2Br����Cl2��Br2��2Cl�� ��������Br2 ����β�� NaOH��Һ H2O2��2Br����2H+��Br2��2H2O �����ԣ�H2O2��Br2��H2SO3 �����ţ������ȿ����Ƿ�μ�����H2SO3��ֻҪ�۲쵽������ɫ������ͬʱ��������ɫ�仯������֤��Br2������H2SO3 H2SO3��ʣ�ࣨH2O2Ũ��С��Br����H2O2��Ӧ�������ض��ɣ�
��������
(1)A������ˮ�����廯����Һ�з��� ��������ԭ��Ӧ����������������Ϊ�嵥�ʣ�A����Һ��Ϊ����ɫ����Ӧ�����ӷ���ʽΪ��2Br-+Cl2=Br2+2Cl-���ʴ�Ϊ��2Br-+Cl2=Br2+2Cl-��
(2)�����ȿ�����A�к���ɫ���Ա�dz��˵���嵥�ʱ�����B�ڣ�B�������ݣ�����������ɫ������˵���嵥����������������������Ȼ������ɰ�ɫ���������Һ��ɫ�����Ա仯����һ��֤�����嵥�ʱ���ԭΪ�����ӣ��ʴ�Ϊ����������Br2��
(3)װ��C��β������װ�ã���Ӧ������������������������Ⱦ�����岻���ŷŵ���������Ҫ������������Һ���գ��ʴ�Ϊ������β����NaOH��Һ��
(4)�����������������Һ�л�����������Ϊ�嵥�ʣ���Ӧ�ķ���ʽ���ݵ����غ㡢ԭ���غ���ƽ��дΪ��H2O2+2Br-+2H+=Br2+2H2O���ʴ�Ϊ��H2O2+2Br-+2H+=Br2+2H2O��
(5)���ݷ�ӦH2SO3+Br2+H2O=H2SO4+2HBr��H2O2+2Br-+2H+=Br2+2H2O��������ԭ��Ӧ�� �������������Դ����������������Ϊ��H2O2��Br2��H2SO3���ʴ�Ϊ��������ΪH2O2��Br2��H2SO3��
(6)����֤����H2SO3��Br2�����˷�Ӧ���������Ƕȣ�H2SO3����������SO42-������ɫ���������� Br2����ԭ����Br-����ɫ���仯�����е�һ���������ĸ��ţ����ڶ�����ȫ����ʵ�������Ѿ�����˵����H2SO3����������Br2�����˷�Ӧ���ʴ�Ϊ�������ţ������ȿ����Ƿ�μ�����H2SO3��ֻҪ�۲쵽������ɫ������ͬʱ��������ɫ�仯������֤��Br2������H2SO3��
��ʵ�����III����ʼʱ��ɫ�����Ա仯��ԭ���ǣ��Ȼ���Ũ��С�����������������ӷ�Ӧ������������ʣ��ȣ��ʴ�Ϊ��H2SO3��ʣ��(H2O2Ũ��С��Br-��H2O2��Ӧ�������ض���)��