��Ŀ����
��10�֣�Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q��W��ɵĻ���������Ȼ������Ҫ�ɷ֣�W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1�U1��l�U2���������ӻ����
��1��W��Ԫ�����ڱ��е�λ���� ���� �塣
��2����ҵ�ϳ�XQ3�Ƿ��ȷ�Ӧ�����д�ʩ�У����ܼӿ췴Ӧ���ʣ��������ԭ���������� ������д��ţ�
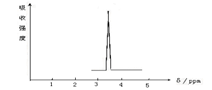
��3��2.24 L����״����XQ3��200 mL l mol/L QXY3��Һ���պ�������Һ������Ũ�ȴӴ�С��˳���� ���������ӷ��ű�ʾ��
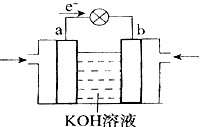
��4��WQ4Y��Y2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ������ͼ��ʾ��a���ĵ缫��Ӧ�� ��
��5����֪��W��s��+Y2��g��=WY2��g���� H=��393.5kJ/mol
H=��393.5kJ/mol
WY��g��+ Y2��g��=WY2��g����
Y2��g��=WY2��g����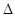 H=��238.0kJ/mol���� 24g W��һ������Y2��Ӧ���ų�����362.5 kJ�����ò���ɷּ����ʵ���֮��Ϊ ��
H=��238.0kJ/mol���� 24g W��һ������Y2��Ӧ���ų�����362.5 kJ�����ò���ɷּ����ʵ���֮��Ϊ ��
��6��X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��1��W��Ԫ�����ڱ��е�λ���� ���� �塣
��2����ҵ�ϳ�XQ3�Ƿ��ȷ�Ӧ�����д�ʩ�У����ܼӿ췴Ӧ���ʣ��������ԭ���������� ������д��ţ�
| A�������¶� | B��������� | C����XQ3��ʱ�����ȥ | D������Ӧ��ϵ��ѹǿ |
��4��WQ4Y��Y2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ������ͼ��ʾ��a���ĵ缫��Ӧ�� ��

��5����֪��W��s��+Y2��g��=WY2��g����
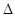 H=��393.5kJ/mol
H=��393.5kJ/molWY��g��+
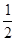 Y2��g��=WY2��g����
Y2��g��=WY2��g����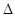 H=��238.0kJ/mol���� 24g W��һ������Y2��Ӧ���ų�����362.5 kJ�����ò���ɷּ����ʵ���֮��Ϊ ��
H=��238.0kJ/mol���� 24g W��һ������Y2��Ӧ���ų�����362.5 kJ�����ò���ɷּ����ʵ���֮��Ϊ ����6��X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��1���ڶ����ڡ��ڢ�A�塣 ��2��D ��3��c(NO3-)��c( H+)��c(NH4+)��c(OH-)
��4��CH4 �C 8e- + 10OH- = CO32- + 7H2O ��5��CO2��CO 1��8.24
��6��Na3N + 4H2O =" 3NaOH" + NH3.H2O
��4��CH4 �C 8e- + 10OH- = CO32- + 7H2O ��5��CO2��CO 1��8.24
��6��Na3N + 4H2O =" 3NaOH" + NH3.H2O
Q��W��ɵĻ���������Ȼ������Ҫ�ɷ֣�����Q��H��W��C���������ų��Ĵ�����Ⱦ����Ҫ��̼��������͵�����������X��N��Y��O��Y��Z���γ�ԭ�Ӹ�����Ϊ1�U1��l�U2���������ӻ����˵��Z����Ԫ�ء�
��2�����ĺϳ��Ƿ��ȵġ������С�Ŀ��淴Ӧ�������¶ȣ�������ƽ��������Ӧ������У���������ƽ��״̬����XQ3��ʱ�����ȥ�ή�ͷ�Ӧ���ʣ���ȷ�Ĵ���D��
��3��0.1molNH3��0.2molHNO3��Ϻ������������Һ�к��е�������NH4NO3��HNO3������������Һ������Ũ�ȴӴ�С��˳����c(NO3-)��c( H+)��c(NH4+)��c(OH-)��
��4�����ݵ��ӵ����������֪a�Ǹ�����Ӧͨ����飬����a���ĵ缫��Ӧ��CH4 �C 8e- + 10OH- = CO32- + 7H2O��
��5�����������Ȼ�ѧ����ʽ�ϲ��ɵõ�W��s��+1/2Y2��g��=WY��g����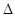 H=��155.5kJ/mol��
H=��155.5kJ/mol��
���24gC��ȫȼ�շų���������787kJ������ȫȼ�շų���������311 kJ����ʵ�ʷų�362.5
kJ�����Բ�����CO��CO2�Ļ�������ʮ�ֽ��淨�ɼ���CO��CO2�����ʵ���֮����

��6��X��Z�ֱ���N��Na���仯�ϼ۷ֱ��ǣ�3�ۺͣ�1�ۣ������仯ѧʽΪNa3N����Ӧ�ķ���ʽΪNa3N + 4H2O =" 3NaOH" + NH3.H2O��
��2�����ĺϳ��Ƿ��ȵġ������С�Ŀ��淴Ӧ�������¶ȣ�������ƽ��������Ӧ������У���������ƽ��״̬����XQ3��ʱ�����ȥ�ή�ͷ�Ӧ���ʣ���ȷ�Ĵ���D��
��3��0.1molNH3��0.2molHNO3��Ϻ������������Һ�к��е�������NH4NO3��HNO3������������Һ������Ũ�ȴӴ�С��˳����c(NO3-)��c( H+)��c(NH4+)��c(OH-)��
��4�����ݵ��ӵ����������֪a�Ǹ�����Ӧͨ����飬����a���ĵ缫��Ӧ��CH4 �C 8e- + 10OH- = CO32- + 7H2O��
��5�����������Ȼ�ѧ����ʽ�ϲ��ɵõ�W��s��+1/2Y2��g��=WY��g����
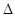 H=��155.5kJ/mol��
H=��155.5kJ/mol�����24gC��ȫȼ�շų���������787kJ������ȫȼ�շų���������311 kJ����ʵ�ʷų�362.5
kJ�����Բ�����CO��CO2�Ļ�������ʮ�ֽ��淨�ɼ���CO��CO2�����ʵ���֮����

��6��X��Z�ֱ���N��Na���仯�ϼ۷ֱ��ǣ�3�ۺͣ�1�ۣ������仯ѧʽΪNa3N����Ӧ�ķ���ʽΪNa3N + 4H2O =" 3NaOH" + NH3.H2O��

��ϰ��ϵ�д�
 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
�����Ŀ
 N2(g)+2H2O(l) ��H="-a" kJ/mol
N2(g)+2H2O(l) ��H="-a" kJ/mol Cu2O+H2������������ӦʽΪ�� ��
Cu2O+H2������������ӦʽΪ�� �� 2H2(g)��O2(g) ��H����484 kJ��mol��1
2H2(g)��O2(g) ��H����484 kJ��mol��1

 CH3OH(g) ��H��
CH3OH(g) ��H��
 2SO3(g)����H < 0 �����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯
2SO3(g)����H < 0 �����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯 ͼ��
ͼ��