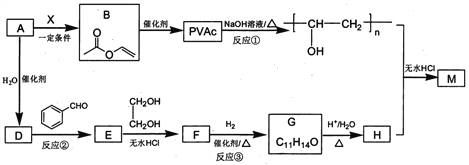
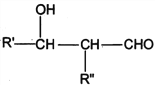
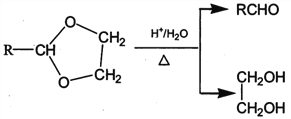
��Ŀ����
����Ŀ����1����CH3OH�Ϳ���Ϊԭ�ϵ�ȼ�ϵ�أ������ΪKOH��Һ���ش��������⣺
�� OH������____________����
�� ������ӦʽΪ__________________________________��
�� �����pH����______________���������١����䣩��
��2��������ĵ�ض������ʽ��е�⣬����a��b��c��d��e��f�缫��Ϊ���Ե缫��ͨ��һ��ʱ��� e������0.064g����
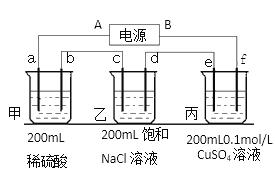
�ٵ�ԴB����____________����
�ڼ׳�a�缫��ӦʽΪ ___________________________________________��
���ҳط�Ӧ��ѧ����ʽ________________________________________________��
���ҳع����ռ�����״�����������Ϊ_____________mL������Һ��pHΪ___________�������£���������Һ����仯��
���𰸡��� CH3OH ��6e-+ 8OH��= 6H2O+CO32�� ��С �� 2H++2e-= H 2�� 2NaCl+2H2O ![]() 2NaOH+Cl2��+H2 �� 44.8 12
2NaOH+Cl2��+H2 �� 44.8 12
��������
��1���ٵ�������������ƶ���
�ڸ�������������Ӧ��
�۵���ܷ�ӦΪ��![]() ��
��
��2������e�����أ���֪e��������ͭ����eΪ��������AΪ������BΪ������a��c��eΪ������b��d��fΪ������
��1���ٵ�������������ƶ�����![]() ������
������
�ڸ�������������Ӧ���״�����������Ӧ����̼������缫��ӦʽΪ��![]() ��
��
�۵���ܷ�ӦΪ��![]() ����Ӧ����������������ҺpH��С��
����Ӧ����������������ҺpH��С��
�ʴ�Ϊ������![]() ����С��
������
��2������e�����أ���֪e��������ͭ����eΪ��������AΪ������BΪ������a��c��eΪ������b��d��fΪ������
��e�����أ���֪e��������ͭ����eΪ��������AΪ������BΪ������
��aΪ�������������������������ŵ������������缫��ӦʽΪ��![]() ��
��
���ҳ���Ϊ���Ե缫����Ȼ�����Һ���ܷ�ӦΪ��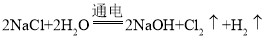 ��
��
�ܸ���e������0.064g��������0.001molCu����ת�Ƶ���0.002mol����Ϊ�Ǵ�����·������ÿ���缫�ĵ�������ͬ������![]() ����֪c������0.001mol
����֪c������0.001mol![]() ��d������0.001mol
��d������0.001mol![]() ���ҳ��в���0.002mol
���ҳ��в���0.002mol![]() �����ҳ��й��ռ���0.002mol���壬0.002mol�����ڱ�״���µ����Ϊ44.8mL���壬
�����ҳ��й��ռ���0.002mol���壬0.002mol�����ڱ�״���µ����Ϊ44.8mL���壬![]() ����pH=12��
����pH=12��
�ʴ�Ϊ������![]() ��
��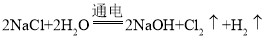 �� 44.8 ��12��
�� 44.8 ��12��

����Ŀ��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ��
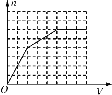
��1�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����������������Ϊ__mL���ζ��յ�ʱ�������ǣ����������һ������ʱ����ƿ����Һ��ɫ��___����30s���ָ�ԭɫ��

��2��ijѧ����������ʵ��ֱ��¼�й��������±���
�ζ����� | ������������ ��Һ�����/mL | 0.1000mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ��� | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
��ѡ�����к����������������������Һ���ʵ���Ũ��(����������4λ��Ч����)��c(NaOH)=__mol��L��1��
��3�����ڴ��������ʹ��������������������Һ��Ũ��ƫ�ߵ���__(����ĸ)��
A.�ζ�ǰ�ζ����������ݣ��ζ�����ʧ B.��ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ����
C.�ζ�ʱ�ﵽ�ζ��յ�ʱ���Ӷ��� D.��ƿȡ��NaOH����Һǰ������ˮϴ��