��Ŀ����
 ���أ�H2NCONH2�����������л����ʣ���Ҫ������[Fe��H2NCONH2��6]��NO3��3[�����������غ�������]��
���أ�H2NCONH2�����������л����ʣ���Ҫ������[Fe��H2NCONH2��6]��NO3��3[�����������غ�������]����1����̬Fe3+�ĺ�������Ų�ʽΪ
��2�����ط�����C��Nԭ�ӵ��ӻ���ʽ�ֱ���
��3��[Fe��H2NCONH2��6]��NO3��3�С�H2NCONH2����Fe����֮�����������
��4��CO2��NH3�ǹ�ҵ���Ʊ����ص���Ҫԭ�ϣ���̬CO2���ɱ����ľ����ṹ��ͼ��ʾ��
��ͭ��Ͻ�ľ����ṹ��ɱ����ƣ�������ΪAu������ΪCu����ͭ��Ͻ�����Au��Cuԭ����֮��Ϊ��
����֪�ɱ������߳�Ϊa pm����þ�����ܶ�Ϊ
���㣺�����ļ���,ԭ�Ӻ�������Ų�,ԭ�ӹ���ӻ���ʽ���ӻ������ж�
ר�⣺��ѧ���뾧��ṹ
��������1��Feԭ�Ӻ��������Ϊ26��ԭ���γ��������Ȱ��ܲ�ߵ�ʧȥ���ӣ��ܲ�Խ�ߵĵ���Խ����ʧȥ��ͬһ�ܲ��а��ܼ��ߵ�ʧȥ���ӣ��ܼ�Խ��Խ����ʧȥ��
ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ�
��2�������صĽṹʽ ��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ�
��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ�
��3��Fe�����пչ�������ط�����Nԭ�Ӻ��й¶Ե��ӣ������γ���λ����
ԭ��������ȣ��۵���������ȵ���Ϊ�ȵ����壬���ô�������д��
��4���ٸ��ݾ�̯�����㾧���к���Au��Cuԭ����Ŀ���ݴ˼��㣻
�ڸ�����=
���㾧�����ܶȣ�
ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ�
��2�������صĽṹʽ
 ��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ�
��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ���3��Fe�����пչ�������ط�����Nԭ�Ӻ��й¶Ե��ӣ������γ���λ����
ԭ��������ȣ��۵���������ȵ���Ϊ�ȵ����壬���ô�������д��
��4���ٸ��ݾ�̯�����㾧���к���Au��Cuԭ����Ŀ���ݴ˼��㣻
�ڸ�����=
| m |
| V |
���
�⣺��1��Feԭ�Ӻ�����26�����ӣ���������Ų�Ϊ1s22s22p63s23p63d64s2��Feԭ��ʧȥ4s�ܼ�2�����ӡ�3d�ܼ�1�������γ�Fe3+��Fe3+�����Ų�ʽΪ1s22s22p63s23p63d5��
ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������N��O��C��
�ʴ�Ϊ��1s22s22p63s23p63d5��N��O��C��
��2�������ط��ӵĽṹʽ ��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��
��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��
��3��Fe�����пչ��������λ����ĿΪ6��Oԭ���ṩ�¶Ե��ӣ�H2NCONH2��Fe����֮���γ���λ����Nԭ������������Ϊ5����Nԭ�Ӽ�1����λ����ɣ������滻ΪSԭ�ӣ���SO3��NO3-��Ϊ�ȵ����壬
�ʴ�Ϊ����λ����SO3�ȣ�
��4����������ΪAu������ΪCu�������к���Auԭ����ĿΪ8��
=1�������к���Cuԭ����ĿΪ6��
=3����ͭ��Ͻ�����Au��Cuԭ����֮��Ϊ1��3��
�ʴ�Ϊ��1��3��
���ڸɱ������к��ж�����̼����ĿΪ8��
+6��
=4�����Ծ������ܶ�Ϊ
g/cm3=
g/cm3���ʴ�Ϊ��
��
ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������N��O��C��
�ʴ�Ϊ��1s22s22p63s23p63d5��N��O��C��
��2�������ط��ӵĽṹʽ
 ��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��
��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3����3��Fe�����пչ��������λ����ĿΪ6��Oԭ���ṩ�¶Ե��ӣ�H2NCONH2��Fe����֮���γ���λ����Nԭ������������Ϊ5����Nԭ�Ӽ�1����λ����ɣ������滻ΪSԭ�ӣ���SO3��NO3-��Ϊ�ȵ����壬
�ʴ�Ϊ����λ����SO3�ȣ�
��4����������ΪAu������ΪCu�������к���Auԭ����ĿΪ8��
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ��1��3��
���ڸɱ������к��ж�����̼����ĿΪ8��
| 1 |
| 8 |
| 1 |
| 2 |
| ||
| (a��10-10)3 |
| 1.76��1032 |
| aNA |
| 1.76��1032 |
| aNA |
���������⿼���������Ų����ɡ��ӻ�������ۡ�������������ȣ��Ѷ��еȣ���3����ע��ȵ�����Ϊԭ��������ȣ��������������۵���������ȵ�����

��ϰ��ϵ�д�
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
�����Ŀ
�����й�������Ӧʵ���˵����ȷ���ǣ�������
| A���Թ�Ҫ��Ũ���������ˮϴ�� |
| B������ˮԡ���ȣ�����ֱ�Ӽ��� |
| C����2%��ϡ��ˮ�е���2%����������Һ�����������Һ |
| D������Ũ����ϴȥ���� |
���з�Ӧ�У�ˮ�Ȳ�����������Ҳ���ǻ�ԭ��������Ӧ��������ԭ��Ӧ���ǣ�������
| A��2Na2O2+2H2O�T4NaOH+O2�� | ||||
B��C+H2O
| ||||
| C��CO2+NH3+H2O�TNH4HCO3 | ||||
| D��2F2+2H2O�T4HF+O2 |
�����������ڵȵ�����һ����ǣ�������
| A��CH4��NH4+ |
| B��N2O4��C2H4 |
| C��CO2��NO2 |
| D��H2O��CH4 |
������ʵ��������������ԭ�����͵��ǣ�������
| A����ơ��ƿ�ǣ�ƿ��ð���������� |
| B���ϳɰ�����ͨ������20MPa��50MPaѹǿ�������ԭ�ϵ������� |
| C����H2��I2��g����HI��g��������ɵ�ƽ����ϵ��ѹ����ɫ��dz |
| D����ҵ��ȡ������Na��l��+KCl��l��?NaCl��l��+K��g����ѡȡ���˵��¶ȣ�ʹK��������ӷ�Ӧ������з������ |
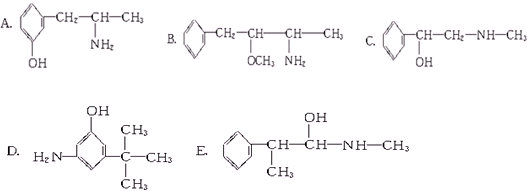
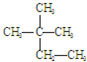 �۰��� ��18O ��
�۰��� ��18O ��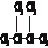 ���� ��16O ��������
���� ��16O ��������