��Ŀ����
��2013?������ģ������˵����ȷ���ǣ�������
������A��pH��ͬʱ����Һ��c��H+��������Ϊ���ᣬ��ҺŨ�����
B��������Һ�ĵ���غ��жϣ�
C����������ʵĵ����Ӱ������ˮ������ط�����
D��Al3+ˮ�⣬�ٽ�ˮ�ĵ��룮
B��������Һ�ĵ���غ��жϣ�
C����������ʵĵ����Ӱ������ˮ������ط�����
D��Al3+ˮ�⣬�ٽ�ˮ�ĵ��룮
����⣺A��pH��ͬʱ����Һ��c��H+��������Ϊ���ᣬ��ҺŨ������к�NaOH��Һʱ����NaOH�����ʵ����ɴ�С��˳���Ǣ٣���=�ڣ���A����
B��NaHCO3��Һ�д��ڵ���غ㣬ӦΪc��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-������B����
C��CH3COOHΪ���ᣬ����̶Ƚ�С������Һ��c��CH3COO-����С������CH3COO-��NH4+��������ˮ�⣬��CH3COONH4��Һ��CH3COO-Ũ��С��CH3COONa����C��ȷ��
D��Al3+ˮ�⣬�ٽ�ˮ�ĵ��룬��Һ��H+��ȫ��ˮ���룬��ˮ�������H+�����ʵ���Ũ��Ϊ10-4mol?L-1����D����
��ѡC��
B��NaHCO3��Һ�д��ڵ���غ㣬ӦΪc��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-������B����
C��CH3COOHΪ���ᣬ����̶Ƚ�С������Һ��c��CH3COO-����С������CH3COO-��NH4+��������ˮ�⣬��CH3COONH4��Һ��CH3COO-Ũ��С��CH3COONa����C��ȷ��
D��Al3+ˮ�⣬�ٽ�ˮ�ĵ��룬��Һ��H+��ȫ��ˮ���룬��ˮ�������H+�����ʵ���Ũ��Ϊ10-4mol?L-1����D����
��ѡC��
���������⿼���Ϊ�ۺϣ��漰������ʵĵ��롢�����ˮ�⣬ע�����Ӱ������ˮ������أ��״���ΪD��ע������ˮ���ԭ����Ӧ�ã�

��ϰ��ϵ�д�
 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�
�����Ŀ

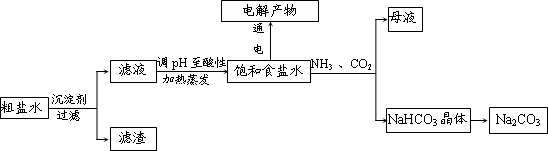
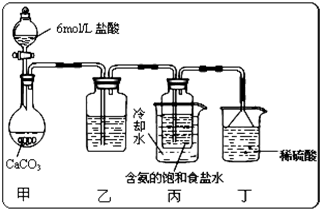
 ��2013?������ģ��ijϡ�����ϡ����Ļ����Һ200mL��ƽ���ֳ����ݣ�������һ��������ͭ�ۣ�������ܽ�9.6g������һ�����������ۣ�������������������������ӵı仯����ͼ��ʾ����֪����ֻ����ԭΪNO���壩�����з�������������ǣ�������
��2013?������ģ��ijϡ�����ϡ����Ļ����Һ200mL��ƽ���ֳ����ݣ�������һ��������ͭ�ۣ�������ܽ�9.6g������һ�����������ۣ�������������������������ӵı仯����ͼ��ʾ����֪����ֻ����ԭΪNO���壩�����з�������������ǣ�������