��Ŀ����
6�������д����ǿ�ѧ��������ѧ֪ʶ�ش��������⣮��1������Ǽ�ͥ����ҩ�����Ƶ�������Ƭ�ϣ���������������������ɫ��
��2��������ֳơ���李�����һ�ֳ��õ��ʣ����������Һ�м�����ɫʯ����Һ����Һ���ɫ���������Һ�����ԡ�������ԡ��������ԡ������ԡ����̬����������ʱ�ܷ�����ѧ��Ӧ���÷�Ӧ�Ļ�����Ӧ�����Ǹ��ֽⷴӦ��
��3��ʳ���к�3%��5%�Ĵ��ᣬ�����г���ʳ����ϴˮ����Mg��OH��2��CaCO3������֪������ˮ���ܵ����CH3COO-��H+��2CH3COOH+CaCO3=��CH3COO��2Ca+H2O+CO2������д��������Mg��OH��2��Ӧ�Ļ�ѧ����ʽ��Mg��OH��2+2CH3COOH=��CH3COO��2Mg+2H2O��
���� ��1�������������ɫ��
��2�������Ϊǿ�������Σ����������ֽⷴӦ���ɰ�����
��3��������������þ��Ӧ���ɴ���þ��ˮ��
��� �⣺��1���������е��ۣ������������ɫ���ʴ�Ϊ����������ɫ��
��2�������Ϊǿ�������Σ�ˮ������ԣ����������ֽⷴӦ���ɰ������ʴ�Ϊ�����ԣ����ֽⷴӦ��
��3��������������þ��Ӧ���ɴ���þ��ˮ����Ӧ�ķ���ʽΪMg��OH��2+2CH3COOH=��CH3COO��2Mg+2H2O���ʴ�Ϊ��Mg��OH��2+2CH3COOH=��CH3COO��2Mg+2H2O��
���� �����ۺϿ���Ԫ�ػ�����֪ʶ�������ڻ�ѧ������Ŀ��飬����������ѧ�������õĿ�ѧ���������ѧϰ�Ļ����ԣ��ѶȲ���ע����ػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
�����Ŀ
10�����ڽ��壬����˵����ȷ���ǣ�������
| A�� | ����ľ۳��ǻ�ѧ�仯 | |
| B�� | ����0.01mol FeCl3����Һ�ƳɵĽ����У��������ӵ���ĿԼΪ6.02��1021�� | |
| C�� | ����Ľ������뽺����е���й� | |
| D�� | NaCl����ȿ��Ƴ���Һ�ֿ��Ƴɽ��� |
11��ij������X�ɿ�����CO2��NaOH��Ӧ���ã�Ϊȷ������ɣ����ʵ�鼰���������ʾ��
��1����2.49g��Ʒ����ͬ����ʵ��ʱ������CO2504mL��
��2����ȡ3.32g������X��Ʒ��300����ȷֽ�����ȫ��Na2CO3���ֽ⣩������CO2112mL����״������ˮ0.45g��������X�Ļ�ѧʽ2Na2CO3•NaHCO3•2H2O��
| �� | �� | �� | �� | |
| ����Һ����� ��mL�� | 30 | 30 | 30 | 30 |
| ��Ʒ��g�� | 3.32 | 4.15 | 5.81 | 7.47 |
| ������̼����� ��mL�� | 672 | 840 | 896 | 672 |
��2����ȡ3.32g������X��Ʒ��300����ȷֽ�����ȫ��Na2CO3���ֽ⣩������CO2112mL����״������ˮ0.45g��������X�Ļ�ѧʽ2Na2CO3•NaHCO3•2H2O��
1��ʵ�����ォ������ˮ�ﱣ�棬�ݴ˶����������������Ʋ⣬����ȷ���ǣ�������
| A�� | ������������Ӧ | B�� | ����ˮ����Ӧ | C�� | ��������ˮ | D�� | ����ˮ�� |
16����NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�� | ÿĦ�����к�10NA������ | |
| B�� | ��״����2.24 L�Ҵ�������Na��Ӧ����H2������0.05NA | |
| C�� | 14 g��ϩ����Hԭ����Ϊ2NA | |
| D�� | 78 g���к��е�̼̼˫������ĿΪ3NA |
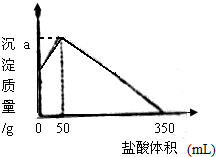 ����NaOH��AlCl3��MgCl2���ֹ�����ɵĻ������������ˮ����ַ�Ӧ��õ�12.6g��ɫ�����������õ���Һ����μ���3mol/L�����ᣬ���ɳ�����������������������Ĺ�ϵ��ͼ��ʾ����������ش��������⣺
����NaOH��AlCl3��MgCl2���ֹ�����ɵĻ������������ˮ����ַ�Ӧ��õ�12.6g��ɫ�����������õ���Һ����μ���3mol/L�����ᣬ���ɳ�����������������������Ĺ�ϵ��ͼ��ʾ����������ش��������⣺