��Ŀ����
����Ŀ�������ᣨH3PO2����һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԣ��ش��������⣺
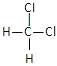
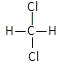
��1����H3PO2��һԪ��ǿ�ᣬд������뷽��ʽ______________________��
��NaH2PO2Ϊ___________������������������ʽ������������Һ��___________��������������������������������������
��H3PO2�У�PԪ�صĻ��ϼ�Ϊ___________��
��2��H3PO2�����ڻ�ѧ��������Ӧ��Ag+��ԭΪ����H3PO2����ΪH3PO4���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ________��
��3��H3PO2�Ĺ�ҵ�Ʒ���:������(P4)��Ba(OH)2��Һ��Ӧ����PH3�����Ba(H2PO2)2����������H2SO4 ��Ӧ��д��������Ba(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ______________________��
���𰸡�H3PO2![]() H++H2PO2- ���� ������ +1 4:1 2P4+3Ba(OH)2+6H2O =2PH3+3Ba(H2PO2)2
H++H2PO2- ���� ������ +1 4:1 2P4+3Ba(OH)2+6H2O =2PH3+3Ba(H2PO2)2
��������
(1)����H3PO2��һԪ��ǿ���֪��H3PO2��������ʣ���Һ�в��ֵ���������ӣ��ݴ�д�����뷽��ʽ���γɵ���Ϊ����ǿ���Σ�ˮ��ʼ��ԣ����ݻ��������ܻ��ϼ�Ϊ0�����PԪ�صĻ��ϼۣ�
(2)���ж�����������ԭ����Ȼ����ݻ��ϼ������غ㣬�����������ͻ�ԭ���ı�����
(3)������ԭ��Ӧ�����ݵ�ʧ�����غ��ԭ���غ���д����ʽ��
(1)��H3PO2��һԪ��ǿ�ᣬ��Һ�в��ֵ���������ӣ���������뷽��ʽΪ��H3PO2![]() H2PO2��+H����
H2PO2��+H����
��H3PO2��һԪ�ᣬֻ�ܵ����1��H����NaH2PO2����ȻH2PO2���к���2��Hԭ�ӣ����Ǿ����ܵ������������NaH2PO2Ϊ���Ρ�H2PO2��Ϊ�������������ӣ��ܹ����ˮ���������H����ʹ����Һ�е�OH����Ũ�ȴ���H����Ũ�ȣ���Һ���������ԣ�
�۸��ݻ��ϼ۴�����ΪO����P�Ļ��ϼ�Ϊx����3��1��x��2��(��2)=0����x=��1��P�Ļ��ϼ�Ϊ+1��
(2)Ag����ԭ��Ag��Ag��Ϊ�����������ϼ۽���1�ۡ�H3PO2����ΪH3PO4��H2PO2����ԭ�����仯�ϼ۴ӣ�1���ߵ���5������4�ۣ����ϼ������غ㣬�������ͻ�ԭ���ı���Ϊ4��1��
(3)PH3��P�Ļ��ϼ�Ϊ��3�����ϼ۴�0���͵���3���ܹ�������3�ۡ�Ba(H2PO2) 2��P�Ļ��ϼ�Ϊ��1��������2��P�����ϼ��ܹ�����2�ۡ�P4�������绯��Ӧ�����ϼ������غ㣬��PH3��Ba(H2PO2) 2�ı���Ϊ2��3���ٸ���ԭ���غ���ƽ����ѧ����ʽΪ2P4+3Ba(OH)2��6H2O =2PH3��3Ba(H2PO2)2��