��Ŀ����
����Ŀ��H2C2O4Ϊ��Ԫ���ᡣ20��ʱ������һ��c��H2C2O4��+ c��HC2O4�C��+ c��C2O42�C��=0.100 mol��L�C1��H2C2O4��NaOH�����Һ����Һ�в����������ʵ���Ũ����pH�ı仯������ͼ��ʾ������ָ����Һ���������ʵ���Ũ�ȹ�ϵһ����ȷ������ ��
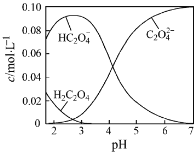
A��pH=2.5����Һ�У�c(H2C2O4)+c(C2O42�C)��c(HC2O4�C)
B��c(Na+)=0.100 mol��L�C1����Һ�У�c(H+)+c(H2C2O4)=c(OH�C)+c(C2O42�C)
C��c(HC2O4�C)=c(C2O42�C)����Һ�У�c(Na+)��0.100 mol��L�C1+c(HC2O4�C)
D��pH=7����Һ�У�c(Na+)��2c(C2O42�C)
���𰸡�BD
��������
���������A������ͼ���֪��pH=2.5ʱ��c(H2C2O4)��c(C2O42��)<c(HC2O42��)���ʴ���B�����������غ㣬c(Na��)=c(H2C2O4)��c(HC2O4��)��c(C2O42��)�����ݵ���غ㣬c(Na��)��c(H��)=c(HC2O4��)��c(OH��)��2c(C2O42��)����ʽ�ϲ����ó�c(H��)��c(H2C2O4)=c(OH��)��c(C2O42��)������ȷ��C��c(Na��)��c(H��)=c(HC2O4��)��c(OH��)��2c(C2O42��)�������c(Na��)=c(H2C2O4��)��2c(C2O42��)��c(OH��)��c(H��) ������õ���c(Na��)=0.1��c(HC2O4��)��c(OH��)��c(H��)��c((H2C2O4)����pH=4ʱ��c(HC2O4��)=c(C2O42��)�����c(H��)>c(OH��)����c(OH��)��c(H��)��c(H2C2O4)<0��c(Na��)<0.1��c(HC2O4��)���ʴ���D����c(Na��)��c(H��)=c(HC2O4��)��c(OH��)��2c(C2O42��)����ΪpH=7�������c(H��)=c(OH��)��c(Na��)=c(HC2O4��)��2c(C2O42��)�����c(Na��)>2c(C2O42��)������ȷ��

 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�����Ŀ���Թ�����Ϊ�����������������V2O5���ǽӴ�����������Ĵ������ӷϷ������л���V2O5�ȱ�����Ⱦ��������������Դ�ۺ����á��Ϸ���������Ҫ�ɷ�Ϊ��
���� | V2O5 | V2O4 | K2SO4 | SiO2 | Fe2O3 | Al2O3 |
��������/% | 2.2~2.9 | 2.8~3.1 | 22~28 | 60~65 | 1~2 | <1 |
������һ�ַϷ��������չ���·�ߣ�
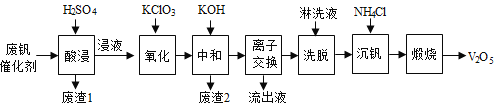
�ش��������⣺
��1���������ʱV2O5ת��ΪVO2+����Ӧ�����ӷ���ʽΪ________________________��ͬʱV2O4ת��VO2+��������1������Ҫ�ɷ���__________________��
��2��������������ʹ3 mol��VO2+��ΪVO2+������Ҫ������KClO3����Ϊ_____________mol��
��3�����к�������֮һ��ʹ����V4O124��ʽ��������Һ�С�������2���к���______________��
��4�������ӽ���������ϴ�����ɼ�ʾΪ��4ROH+ V4O124![]() R4V4O12+4OH��ROHΪǿ���������ӽ�����֬����Ϊ�����ϴ��Ч�ʣ���ϴҺӦ�ó�_____�ԣ�����������������������������
R4V4O12+4OH��ROHΪǿ���������ӽ�����֬����Ϊ�����ϴ��Ч�ʣ���ϴҺӦ�ó�_____�ԣ�����������������������������
��5�����������õ�ƫ����泥�NH4VO3��������д�����������з�����Ӧ�Ļ�ѧ����ʽ____________��