��Ŀ����
����Ŀ����Ҫ��ش��������⣺
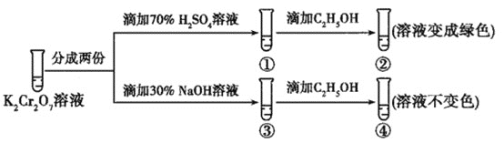
(1)��25�桢101Kpa�£�1gCH3OH(l)ȼ������CO2(g)��H2O(l)ʱ����22.68kJ����CH3OHȼ�յ��Ȼ�ѧ����ʽΪ__________________��
(2)����(1)�з�Ӧԭ����Ƴɼ״�ȼ�ϵ�أ��������ҺΪ20%~30%�� KOH��Һ�����ȼ�ϵ�صĸ����缫��ӦʽΪ___________________��
(3)���Ե缫���400mL2mol/LCuSO4��Һ��һ��ʱ����������1.28g (�ü�������ų�)��������Һ������䣬����Һ��pH Ϊ_____���������ռ����������____mL��д���������ӷ���ʽ��_____
(4)ij�¶��£���Ӧ2NH3(g)![]() N2(g)+3H2(g)�ں����ܱ������дﵽƽ��״̬��(����������������������������)����ijʱ�����������ͨ��һ������������ѧƽ��________�ƶ�����ijʱ���������������ʵ�Ũ�Ⱦ�����Ϊԭ����2������ѧƽ��________�ƶ���
N2(g)+3H2(g)�ں����ܱ������дﵽƽ��״̬��(����������������������������)����ijʱ�����������ͨ��һ������������ѧƽ��________�ƶ�����ijʱ���������������ʵ�Ũ�Ⱦ�����Ϊԭ����2������ѧƽ��________�ƶ���
���𰸡�2CH3OH(1)+3O2(g)=2CO2(g)+4H2O(1) ��H=-1451.52kJ/mol CH3OH-6e-+8OH-= CO32-+6H2O 1 224 2Cu2++2H2O=2Cu+O2+4H+ ���� ����
��������
(1) 1gCH3OH(l)��![]() mol��ȼ������CO2(g)��H2O(l)ʱ����22.68kJ����1mol�״���ȫȼ���ͷŵ�����Ϊ22.68kJ��32=725.76kJ��
mol��ȼ������CO2(g)��H2O(l)ʱ����22.68kJ����1mol�״���ȫȼ���ͷŵ�����Ϊ22.68kJ��32=725.76kJ��
(2) ȼ�ϵ�صĸ����缫ʧ��������Һ�е����������ӷ�Ӧ����̼������Ӻ�ˮ��
(3)���Ե缫���CuSO4��Һʱ������ͭ���ӵõ������ɵ���Cu���������������Ӻ�������
(4)������������ԭ���жϡ�
(1) 1gCH3OH(l)��![]() mol��ȼ������CO2(g)��H2O(l)ʱ����22.68kJ����1mol�״���ȫȼ���ͷŵ�����Ϊ22.68kJ��32=725.76kJ��ȼ���ȵķ���ʽΪCH3OH(l)+
mol��ȼ������CO2(g)��H2O(l)ʱ����22.68kJ����1mol�״���ȫȼ���ͷŵ�����Ϊ22.68kJ��32=725.76kJ��ȼ���ȵķ���ʽΪCH3OH(l)+![]() O2(g)= CO2(g) + 2H2O(1) ��H=-725.76kJ/mol��
O2(g)= CO2(g) + 2H2O(1) ��H=-725.76kJ/mol��
(2) ȼ�ϵ�صĸ����缫ʧ��������Һ�е����������ӷ�Ӧ����̼������Ӻ�ˮ���缫��ӦʽΪCH3OH-6e-+8OH-= CO32-+6H2O��
(3)���Ե缫���CuSO4��Һʱ������ͭ���ӵõ������ɵ���Cu������1.28g ����0.02mol��ת��0.04mol���ӣ�����������0.04mol�����Ӻ�0.01mol����������c(H+)=![]() =0.1mol/L����pH=1��0.01mol����������µ����Ϊ224mL���缫��Ӧ�������ӷ���ʽΪ2Cu2++2H2O=2Cu+O2+4H+��
=0.1mol/L����pH=1��0.01mol����������µ����Ϊ224mL���缫��Ӧ�������ӷ���ʽΪ2Cu2++2H2O=2Cu+O2+4H+��
(4) ��Ӧ�ﵽƽ��״̬����ͨ��һ����������������Ũ������ƽ��������С�ķ����ƶ����������ƶ���ijʱ���������������ʵ�Ũ�Ⱦ�����Ϊԭ����2�����൱������ѹǿ����С���Ϊԭ����![]() ����ƽ���������������С�ķ����ƶ����������ƶ���
����ƽ���������������С�ķ����ƶ����������ƶ���

����Ŀ��ijС��ͬѧ��FeCl3��KI�ķ�Ӧ����̽����
������̽���������½����±�����ʵ�顣
��� | ���� | ���� |
ʵ��� | ȡ5 mL 0.1 mol��L-1 KI��Һ���μ�0.1 mol��L-1FeCl3��Һ5��6��(�����ҺpH=5) | ��Һ��Ϊ�ػ�ɫ |
ʵ��� | ȡ2 mLʵ���Ӧ�����Һ���μ�2��0.1 mol��L-1 KSCN��Һ | ��Һ�ʺ�ɫ |
��1��֤��ʵ�������I2���ɣ�������Լ�Ϊ __________��
��2��д��ʵ���Ӧ�����ӷ���ʽ��_________________��
��3���������ʵ���������֤��Fe3+��I-�������淴Ӧ��ԭ����_____________________��
������̽����20 min������۲�ʵ������ʵ�����Һ�ػ�ɫ���ʵ�����Һ��ɫ��dz��
��4����֪�����Խ�ǿ�������£�I-�ɱ���������ΪI2���ʼ�ͬѧ������裺�÷�Ӧ�����¿�����I-����ΪI2��ʹʵ�������Һ�ػ�ɫ�����ͬѧ���ʵ�飺________��20 min����Һ��������֤���ü��費������������Һ������������������_____________(д������)��
��5����ͬѧ�������Ͽ�֪��FeCl3��KI�ķ�Ӧ��ϵ�л�����I- + I2![]() I3-��I3-���غ�ɫ���������ϴ�ƽ���ƶ�ԭ������ʵ�����20 min����Һ��ɫ��dz��ԭ��____________��
I3-��I3-���غ�ɫ���������ϴ�ƽ���ƶ�ԭ������ʵ�����20 min����Һ��ɫ��dz��ԭ��____________��
��6����ͬѧ���20 min���ʵ���������������裺FeCl3��KI�ķ�Ӧ��I-��I2�ķ�Ӧ�ﵽƽ����Ҫһ��ʱ�䣬�п���20 min֮ǰ��δ�ﵽƽ�⡣Ϊ��֤�ü��裬��ͬѧ��4֧�Թܽ���ʵ�飬�õ�����ɫ��dz������ĸ���ɫ��Һ��ϵ������ʵ�鷽��Ϊ__________________��