��Ŀ����
����Ŀ��������ʾ�����ô������ƴ�����ˮ�еİ�����NH3����ʹ��ת��Ϊ������ȥ������Ҫ��Ӧ���£�
�� NH3��aq��+ HClO��aq���T NH2Cl��aq��+ H2O��l��
�� 2NH2Cl��aq��+ HClO��aq���T N2��g��+ H2O��l��+ 3HCl��aq��
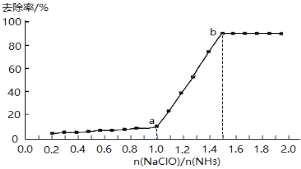
��1���������������������£���һ������ˮ����εμӴ���������Һ������ȥ������n(NaClO)/n(NH3)�ı仯�������£�
��.����NH2Cl�е�Ԫ�صĻ��ϼ���____________��
��.a-b��䣬��Һ�а���ȥ����Ѹ����ߵ�ԭ����__________��
��2����.��Ӧ�٢���HClO ����Դ�û�ѧ���������__________��
��.ʵ���ã���ˮ��pH�백��ȥ������ͼ��ʾ��
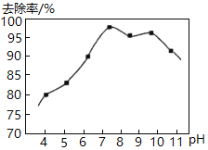
pH�ϸ�ʱ������ȥ�����½���ԭ����__________��
pH�ϵ�ʱ������ȥ����Ҳ�½������ܵ�ԭ����__________��
��3����������ԭ��ȥ����ˮ�еİ������ɽ����ͼ��ʾ�ĵ�ⷨ��
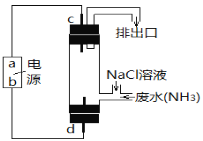
��. a��Ϊ__________��
��. d����ӦʽΪ__________��
���𰸡� -1 c(NaClO)������ˮ��ƽ�������ƶ���ʹc(HClO)������NH3������ΪN2�ٶȼӿ� ClO- + H2O ![]() HClO + OH- pH�ϴ�ʱ��c(OH-)�ϴ�������NaClOˮ����c(HClO)��С��������������ȥ���ʽ��� pH��Сʱ��c(H+)�ϴ����ٽ�NaClOˮ����c(HClO)̫����HClO�ֽ���ȥ���ʽ��� ���� Cl-- 2e- + H2O = H+ + HClO
HClO + OH- pH�ϴ�ʱ��c(OH-)�ϴ�������NaClOˮ����c(HClO)��С��������������ȥ���ʽ��� pH��Сʱ��c(H+)�ϴ����ٽ�NaClOˮ����c(HClO)̫����HClO�ֽ���ȥ���ʽ��� ���� Cl-- 2e- + H2O = H+ + HClO
��������
(1)����HΪ+1�ۣ�ClΪ-1�ۣ����������������ϼ۵ĵ�����Ϊ0����NԪ�صĻ��ϼ�Ϊ0-(+1)��2-(-1)=-1���ʴ�Ϊ��-1��
����a-b��䣬��Һ�а���ȥ����Ѹ����ߵ�ԭ����c(NaClO)����ˮ��ƽ�������ƶ���ʹc(HClO)����NH3������ΪN2�ٶȼӿ죬�ʴ�Ϊ��c(NaClO)����ˮ��ƽ�������ƶ���ʹc(HClO)����NH3������ΪN2�ٶȼӿ죻
(2)��Ӧ�٢���HClO����Դ�û�ѧ���������ClO-+H2OHClO+OH-���ʴ�Ϊ��ClO-+H2OHClO+OH-��
����pH�ϴ�ʱ��c(OH-)�ϴ�����NaClOˮ�⣬c(HClO)��С��������������ȥ���ʽ��ͣ�pH��Сʱ��c(H+)�ϴٽ�NaClOˮ�⣬c(HClO)̫����HClO�ֽ⣬ȥ���ʽ��ͣ��ʴ�Ϊ��pH�ϴ�ʱ��c(OH-)�ϴ�����NaClOˮ�⣬c(HClO)��С��������������ȥ���ʽ��ͣ�pH��Сʱ��c(H+)�ϴٽ�NaClOˮ�⣬c(HClO)̫����HClO�ֽ⣬ȥ���ʽ��ͣ�
(3)��ͼ��֪�����ʱCl-ʧȥ����ת��ΪHClO��HClO�������������ɵ�������d���ӵ�Դ����Ϊ��������֪aΪ������dΪ������������ӦΪCl--2e-+H2O=H++HClO���ʴ�Ϊ��������Cl--2e-+H2O=H++HClO��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����һ���¶��£�������X������Y��0.16mol����10L�����ܱ������У�������ӦX(g)+Y(g)![]() 2Z(g)��H<0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ���������±�������˵����ȷ����
2Z(g)��H<0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ���������±�������˵����ȷ����
t/min | 2 | 4 | 7 | 9 |
(Y)/mol | 0.12 | 0.11 | 0.10 | 0.10 |
A. Ӧǰ2min��ƽ������v(Z)=2.0x10-3mol(L��min)
B. �����������䣬�����¶ȣ���Ӧ�ﵽ��ƽ��ǰv(��)>v(��)
C. ���¶��´˷�Ӧ��ƽ�ⳣ��K=1.44
D. �����������䣬�ٳ���0.2molZ��ƽ��ʱX�������������