��Ŀ����
����Ŀ����ѧѡ��5:�л���ѧ����
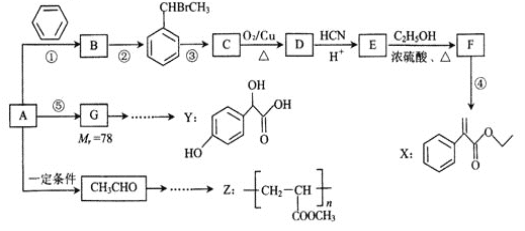
�л���H(C25H44O5)��һ���������Ƹ�Ѫѹ����ҩ���ҵ���Ե��ۡ���AΪ����ԭ�Ϻϳ�H��·������ͼ��ʾ��

��֪:��i����A������ͼ�е�����ʺɱ�Ϊ72��B�����к˴Ź���������2�����������Ϊ9:2��
(ii)  ��
��
(iii)��Ӧ���з�����Ӧ��E��G���ʵ���֮��Ϊ4:1��
(1)A�ķ���ʽΪ_____��B��������_______��C�Ľṹ��ʽΪ_______��
(2)��Ӧ����������______��F�еĹ�����������_______��
(3)д����Ӧ���Ļ�ѧ����ʽ��___________��
(4)E�ж���ͬ���칹�壬������������������ͬ���칹�干��____�֣��˴Ź���������4��������ʽṹ��ʽΪ__________��
���ܷ���������Ӧ �����뵥���Ʒ�����Ӧ
(5)1��3-����ϩ��һ����Ҫ����ԭ�ϣ�����ȩΪ����ԭ�Ͽ��Ƶø�������д����Ӧ��ת������ͼ��__________________________��
���𰸡� C5H12 2��2-����-1-�ȱ��� (CH3)3CCH2OH �ӳɷ�Ӧ �ǻ���ȩ�� (CH3)3CCH2Cl+NaOH![]() (CH3)3CCH2OH+NaCl 12
(CH3)3CCH2OH+NaCl 12 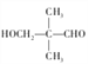 ��
�� CH3CHO
CH3CHO![]() CH3CHOHCH2CHO
CH3CHOHCH2CHO![]() CH3CHOHCH2CH2OH
CH3CHOHCH2CH2OH![]() CH2==CH��CH==CH2
CH2==CH��CH==CH2
�������������������A������ͼ�е�����ʺɱ�Ϊ72��������A����Է�������Ϊ72�������ʽΪCxHy����12x+y=72��y��2x+2�����x=5��y=12������A�ķ���ʽΪC5H12�������̿�֪BΪ±����������ΪB�����к˴Ź���������2�����������Ϊ9:2������B�Ľṹ��ʽΪ(CH3)3CCH2Cl�������̿�֪CΪ����DΪȩ��EΪ���ᣬ��C�Ľṹ��ʽΪ(CH3)3CCH2OH��D�Ľṹ��ʽΪ(CH3)3CCHO��E�Ľṹ��ʽΪ(CH3)3CCOOH��������֪��ii��������̿ɵã�CH3CHO��HCHO�����ӳɷ�Ӧ�����ɺ��ǻ���ȩ�����л���F��F��H2�ӳ�����G��ֻ���ǻ�������Ϊ��Ӧ���з�����Ӧ��E��G���ʵ���֮��Ϊ4:1������F�Ľṹ��ʽΪC(CH2OH)3CHO��G�Ľṹ��ʽΪC(CH2OH)4��
��1�������������ɵã�A�ķ���ʽΪC5H12��B�Ľṹ��ʽΪ(CH3)3CCH2Cl������Ϊ��2��2-����-1-�ȱ�����C�Ľṹ��ʽΪ(CH3)3CCH2OH��
��2��������֪��ii��������̿ɵã�CH3CHO��HCHO�����ӳɷ�Ӧ�����ɵ�F�к����ǻ���ȩ����
��3������������֪��BΪ(CH3)3CCH2Cl��CΪ(CH3)3CCH2OH����B����ˮ�ⷴӦ����C����ѧ����ʽΪ��(CH3)3CCH2Cl+NaOH![]() (CH3)3CCH2OH+NaCl��
(CH3)3CCH2OH+NaCl��
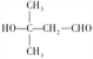
��4��EΪ(CH3)3CCOOH��E��ͬ���칹���У����ܷ���������Ӧ�������ȩ���������뵥���Ʒ�����Ӧ�������ǻ������Է����������У�CH3CH2CH2CH(OH)CHO��CH3CH2CH(OH)CH2CHO��CH3CH(OH)CH2CH2CHO��CH2(OH)CH2CH2CH2CHO��CH3CH2(CH3)C(OH)CHO��CH3CH(OH)CH(CH3)CHO��CH2(OH)CH2CH(CH3)CHO��CH3CH2CH(CH2OH)CHO��CH(CH3)2CH(OH)CHO��(CH3)2C(OH)CH2CHO��CH2(OH)C(CH3)CH2CHO��(CH3)2C(CH2OH)CHO����12�֣����к˴Ź���������4��������ʽṹ��ʽΪ(CH3)2C(OH)CH2CHO��(CH3)2C(CH2OH)CHO��
��5��������֪��ii��������ȩΪ����ԭ���Ƶ�1��3-����ϩ��ת������ͼΪ��CH3CHO![]() CH3CHOHCH2CHO
CH3CHOHCH2CHO![]() CH3CHOHCH2CH2OH
CH3CHOHCH2CH2OH![]() CH2=CHCH=CH2��
CH2=CHCH=CH2��

����Ŀ����֪25��ʱһЩ�������ʵ��ܶȻ��������£�
��ѧʽ | Zn(OH)2 | ZnS | AgCl | Ag2S | MgCO3 | Mg(OH)2 |
�ܶȻ� | 5��10-17 | 2.5��10-22 | 1.8��10-10 | 6.3��10-50 | 6.8��10-6 | 1.8��10-11 |
�����ϱ����ݣ��ж����л�ѧ����ʽ����ȷ����
A. 2AgCl+Na2S�T2NaCl+Ag2S
B. MgCO3+H2O![]() Mg(OH)2+CO2��
Mg(OH)2+CO2��
C. ZnS+2H2O�TZn(OH)2+H2S��
D. Mg(HCO3)2+2Ca(OH)2�TMg(OH)2��+2CaCO3��+2H2O