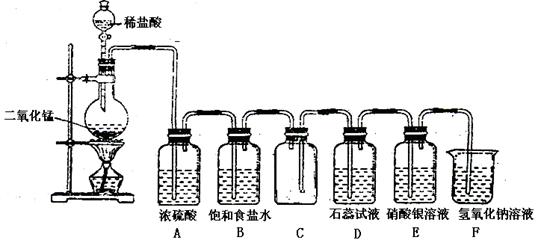
��Ŀ����
Ϊ̽���������Ƶ����ȶ��ԣ�ij�о���ѧϰС�齫��ˮ�������Ƹ���������
�ȣ����������Ⱥ�Ĺ�����������ͼ��ʾ��ʵ��װ�ý���ʵ�顣��ش������й����⣺
��1���������ϣ���ˮ�������Ƹ����������ȵ�600��ſ�ʼ�ֽ⣬�ҷֽ����ֻ������
������һ�ֹ��塣��������¶ȵ���600�棬��������ȴ����������л����μ�ϡ������
�������ڵμ�ϡ��������������� �����ʵ���Ũ�ȱ仯����Ϊ ��
�����ʵ���Ũ�ȱ仯����Ϊ ��
��2����������¶�Ϊ700�棬��������ȴ����������л����μ�ϡ�������������۲쵽��ƿ�г��ֵ���ɫ���������д������ݲ�������Ӧ���ɵ���ɫ���������ӷ���ʽΪ ����ʱ��B��C��װ���п��ܹ۲쵽������Ϊ ��
��3���ڣ�2���еμ������������ƿ�ڳ� �⣬��������һ��Ũ�Ƚϴ�������ӡ�Ϊ����������ӣ���ȡ������������ˮ�����Һ�������Ǽ���������ӵ�����ʵ�鷽������Ϊ�����ķ����� ����ס����ҡ�������˵����һ������������ԭ�� ��
�⣬��������һ��Ũ�Ƚϴ�������ӡ�Ϊ����������ӣ���ȡ������������ˮ�����Һ�������Ǽ���������ӵ�����ʵ�鷽������Ϊ�����ķ����� ����ס����ҡ�������˵����һ������������ԭ�� ��
�����ף�ȡ����������Һ���Թ��У��ȼ�ϡ ���ټ�
���ټ� ��Һ���а�ɫ�������ɣ�֤�������Ӵ��ڡ�
��Һ���а�ɫ�������ɣ�֤�������Ӵ��ڡ�
�����ң�ȡ����������Һ���Թ��У��ȼ�ϡHCl���ټ� ��Һ���а�ɫ�������ɣ�֤�������Ӵ��ڡ�
��Һ���а�ɫ�������ɣ�֤�������Ӵ��ڡ�
��4��д�� ������ȵ�600�����Ϸֽ�Ļ�ѧ����ʽ ��
������ȵ�600�����Ϸֽ�Ļ�ѧ����ʽ ��
��1������������С ��2�֣�
��2��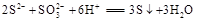 ��2�֣���
��2�֣���
B��Ʒ����Һ��ɫ��C�������Ա仯�������ݣ�2��,���B�������Ա仯�������ݣ�C�в�����ɫ���������� ��B��Ʒ����Һ��ɫ��C�в�����ɫ���������÷֣���
��3���ң�1�֣��������ȼ������ǿ�����Ե�ϡ �����ܽ�
�����ܽ�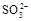 ������
������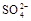 �������ж��Ƿ�����
�������ж��Ƿ�����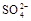 ��2�֣���
��2�֣���
��4��4Na2SO3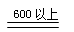 Na2S +3Na2SO4��2�֣�
Na2S +3Na2SO4��2�֣�
���������������1�������¶ȵ���600�棬��������û�зֽ⣬����ϡ���ᷴӦ�ֲ����У�SO32-+H+=HSO3-
HSO3-+H+=SO2��+H2O����Ũ������������٣���2�������������ȷֽ⣬����������ԭ��Ӧ����Ӧ����ʽΪ4Na2SO3 = Na2S +3Na2SO4������������ֵĻ�ɫ����ӦΪS���ʣ������������µ�SO32-��S2-�Ĺ��з�Ӧ������������Ӧ��SO2��H2S�������߲����棬�����ղ���������ֻ��������һ�֣�����ΪB��Ʒ����Һ��ɫ��C�������Ա仯�������ݣ�����ΪSO2����B�������Ա仯�������ݣ�C�в�����ɫ����������ΪH2S������3�������ȼ������ǿ�����Ե�ϡ �����ܽ�
�����ܽ�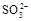 ������
������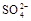 �������ж��Ƿ�����
�������ж��Ƿ�����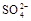 .��
.��
���㣺���黯ѧʵ���й����⡣

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���ʵ�����п�����ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�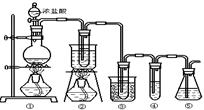
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15mL30��KOH��Һ����������ˮԡ�У��۵��Թ���ʢ��15mL 8%NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
��1����ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��______����д�������ƣ�����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_________����д���б����ĸ���ľ���װ�á�
| A����ʯ�� | B������ʳ��ˮ | C��Ũ���� | D������̼��������Һ |
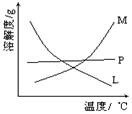
��3����ʵ������ȡ�������Ƶ����ӷ���ʽ�ǣ� ��
��4��ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ___ɫ | ������ˮ��Ӧ���ɵ�H+ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | ______________________________________ |
| Ȼ����Һ����ɫ��Ϊ___ɫ | _________________________________________ |
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��гּ������������ԣ���
��1���Ʊ�����ѡ�õ�ҩƷΪ����������̺�Ũ���ᣬ����ص����ӷ�Ӧ����ʽΪ ��
װ��B�б���ʳ��ˮ��������_________��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е����� ��
��2��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η��� ��
| | a | b | c | d |
| I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��3�����װ��D��E��Ŀ���DZȽ��ȡ��塢�ⵥ�ʵ�������ǿ��������D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ__________ɫ��˵�� ��
��������װ��D��������Һ����װ��E�У����۲쵽�������� ��
��4��װ��F����������NaOH��Һ�������ȣ���д����Ӧ�����ӷ���ʽ ��
�ߴ��ȵ������ǵ��͵����ǽ������ϣ����Ʊ��뵼�����Ҫ���ϣ����ķ��ֺ�ʹ��������������һ�������������ߴ���ͨ�������·����Ʊ�����̼�ڸ����»�ԭ���������Ƶôֹ裨��Fe��Al��B��P�����ʣ����ֹ���������Ӧ�������Ȼ��裨��Ӧ�¶�Ϊ450��500�棩�����Ȼ��辭�ᴿ����������ԭ�ɵøߴ��衣������ʵ�����Ʊ����Ȼ����װ��ͼ��
�����Ϣ��a�����Ȼ�����ˮ����ˮ�⣻b���������������ڸ����¾���������ֱ�ӻ���������Ӧ���Ȼ��c���й����ʵ������������±���
| ���� | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
| �е�/�� | 57.7 | 12.8 | �� | 315 | �� |
| �۵�/�� | ��70.0 | ��107.2 | �� | �� | �� |
| �����¶�/�� | �� | �� | 180 | 300 | 162 |
��ش��������⣺
��1������e������Ϊ____________��װ��A��f�ܵ�������_______________________________________�����з�����Ӧ�����ӷ���ʽΪ_____ ____________________________________ _______��
��2��װ��B�е��Լ���____________��
��3��ijѧϰС���������������ʵ�鷽���������ף�g��װ�â����ң�g��װ�â��Ǽ�������������������װ�þ��в���֮�����������ۺ���д�±���
| ���� | ����֮�� |
| �� | |
| �� | |
��4����������3�������ۻ����ϣ������һ������������___________ ________ ��
��5��ͨ������������װ����ȡ���ռ����Ĵֲ����ͨ���������ƶ�����õ��ߴ������Ȼ��裬�����IJ������У�����Ԫ������ܻ����е�����Ԫ���� ����дԪ�ط��ţ���
ij��ѧС��������װ�ó�ȡ�ռ����������������о������ʡ�������������⡣
��1��װ��A�з�����Ӧ�����ӷ���ʽΪ_______________________________��
��2��������������������ӿڵ�����˳��Ϊa��___________________��g��
��3��װ��B��Ũ�����������__________________________��װ��C���Լ������___________________________________��
��4��ijͬѧ��Ϊ��������ȱ��β������װ�ã���������ķ����л�����װ�ò�ע���Լ���
| |
��5��װ��ȡ������ͨ����ͼ��ʾװ���У���װ����Һ�о��������Եĺ���������_______�����֤����װ����FeCl2��Һ��Cl2�����˷�Ӧ����ֻ�ش���Ҫ���Լ�������_ ��

Ϊ̽��ij��̼�Ͻ���Ũ�����ڼ��������µķ�Ӧ�IJ��ֲ��ﲢ�ⶨ��̼�Ͻ�����Ԫ�ص�����������ij��ѧ�С���������ͼ��ʾ��ʵ��װ�ã����������ʵ��̽����
|
|

 ��1����Բ����ƿ�м���m g��̼�Ͻ𣬲��������Ũ���ᣬδ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ��ٳ����£�Fe��Ũ�����жۻ����� ��
��1����Բ����ƿ�м���m g��̼�Ͻ𣬲��������Ũ���ᣬδ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ��ٳ����£�Fe��Ũ�����жۻ����� ����2����Ӧһ��ʱ���A���ݳ������������Ȼ�Ͽ죬����Ӧ�¶Ƚϸ��⣬�����ܵ�ԭ���� ��
��3��װ��B�������� ��
��4����ͬѧ�۲쵽װ��C���а�ɫ�������ɣ����ó���ʹ����ʯ��ˮ����ǵ������Ƕ�����̼��
װ��A���ܲ���������̼�Ļ�ѧ����ʽΪ ��
��5����ͬѧ��Ϊ��ͬѧ�Ľ����Ǵ���ģ�����ΪΪ��ȷ�϶�����̼�Ĵ��ڣ�����װ��B-C֮������װ��M��װ��E��F��ʢ�ŵ��Լ��ֱ��� �� ��
����ʵ���۲쵽װ��F�е������� ��
��6����Щͬѧ��Ϊ�Ͻ�����Ԫ�ص�������������KMnO4��Һ���ⶨ��
��5Fe2+ + MnO4��+ 8H+ ==5Fe3+ + Mn2+ + 4H2O����
�ⶨ��Ԫ������������ʵ�鲽�����£�
I������ƿA�м�������Ļ�ԭ��ʹ��Һ�е�Fe3+��ȫת��ΪFe2+�����ˣ��õ���ҺB��
II������ҺBϡ��Ϊ250 mL��
III��ȡϡ��Һ25.00 mL����Ũ��Ϊc mol��L��1������KMnO4��Һ�ζ������εζ�ʵ������KMnO4��Һ�����ƽ��ֵΪV mL��
�ٲ�����У�����ҺBϡ��Ϊ250 mL��Ҫ�õ��IJ����������ձ�������������ͷ�ι��⣬������Ҫ�õ����� ��
�ڱ�ͬѧ��������еζ���ʽ���г�����ʡ�ԣ������������ ��������ĸ��ţ�

�۵ζ������� �����Ҫ������Ҫ��������ָʾ��������Ҫ��Ӧ�����ָʾ���� ��
����̼�Ͻ�����Ԫ�ص���������Ϊ ��
�±����������У�������ͼ����һ��ת����ϵ��ѡ���ǣ� ��
| ѡ�� | X | Y | Z |
| A | Na | NaOH | NaHCO3 |
| B | Cu | CuSO4 | Cu��OH��2 |
| C | C | CO | CO2 |
| D | Si | SiO2 | H2SiO3 |