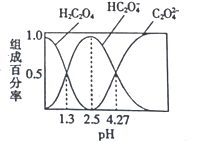
��Ŀ����
����Ŀ����ͼ����ѧ��ѧ�г������ʵ�ת����ϵ���������ʺͷ�Ӧ������ȥ��
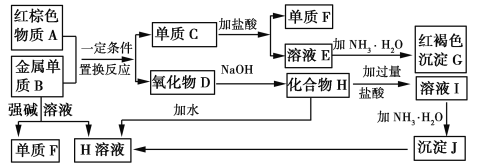
��ش��������⣺
(1)����A�Ļ�ѧʽΪ____________________��
(2)д������B��ǿ����Һ��Ӧ�����ӷ���ʽ_______________��������D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ________________________________��
(3)��ҺE�м��백ˮʱ�������ɰ�ɫ����L��L���ձ�Ϊ���ɫ����G��д��L��ΪG�Ļ�ѧ��Ӧ����ʽ__________________________________��
(4)��ҺE���ڷ������ױ��ʣ�д��������ҺE�Ƿ���ʵ�ʵ�������������__________��Ϊ�˷�ֹ��ҺE�ı��ʣ�������Һ�м���___________________��
(5)��ҺI����������������____________��
���𰸡�Fe2O3 2Al+2OH-+2H2O=2AlO2-+3H2�� Al2O3+2NaOH=2Na AlO2+H2O 4Fe(OH)2+O2+2H2O=4Fe(OH)3 ȡ��ҺE�������Թ��У��μӼ���KSCN��Һ������Һ���ɫ��֤����ҺE�Ѿ����� ���� Na+��Al3+
��������
��������B��ǿ�Ӧ�õ�����F��H����BΪAl��FΪH2��HΪƫ�����Σ�GΪ���ɫ��������GΪFe(OH)3������ɫ����A�����Al�����û���Ӧ���ɵ���C��������D����AΪFe2O3��CΪFe��DΪAl2O3����ת����ϵ��֪��EΪFeCl2��HΪNaAlO2����ҺIΪNaCl��HCl��AlCl3����JΪAl(OH)3���ݴ˽����
������������֪����A��Fe2O3��BΪAl��CΪFe��DΪAl2O3��EΪFeCl2��FΪH2��GΪFe(OH)3��HΪNaAlO2��IΪNaCl��HCl��AlCl3����JΪAl(OH)3��
(1)����ɫ����A��Fe2O3��
(2)Al��NaOH��Һ��Ӧ������ƫ�����ƺ���������Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����������D��Al2O3��Al2O3��NaOH��Һ��Ӧ����ƫ�����ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪAl2O3+2NaOH=2Na AlO2+H2O��
(3)��ҺE ��FeCl2����FeCl2����Һ�м��백ˮʱ�����߷�Ӧ�����ɰ�ɫ����Fe(OH)2��Fe(OH)2���ȶ������ܽ�����Һ�е���������ΪFe(OH)3���������ձ�Ϊ���ɫ����Fe(OH)3���� Fe(OH)2��ΪFe(OH)3�Ļ�ѧ��Ӧ����ʽ��4Fe(OH)2+O2+2H2O=4Fe(OH)3��
(4)��ҺFeCl2���ڷ������ױ��ʣ�������ʣ������FeCl3�����������ʣ���ͨ����E����Һ�еμӼ���KSCN��Һ���飬����Һ��Ϊ��ɫ��֤����ҺE�Ѿ����ʣ�Ϊ�˷�ֹ��ҺE�ı��ʣ�������Fe3+�������ԣ�����Һ�м���Fe�ۣ�������Ӧ��2Fe3++Fe=3Fe2+��ʹ��Һ��ԭ��
(5)������D��Al2O3��Al2O3������NaOH��Һ��Ӧ���õ�������H��NaAlO2����H�м���������ᣬ������Ӧ��NaAlO2+4HCl=NaCl+AlCl3+2H2O������I����������������Na+��Al3+��

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�