��Ŀ����
����Ŀ����1��X��Y��Z����Һ��Ľ���pH��ͼ�������ж���ȷ����___________��

A��Xһ�������ǿ����������Һ B��Yһ����90��ʱ�Ĵ�ˮ
C��YҺ��һ�������� D��Z������Na2SiO3��Һ
��2�����ʵ���Ũ����ͬ��������Һ����NH4Cl�ڰ�ˮ��NH4HSO4��c(NH4+)��С˳����ȷ��
��___________��
A����>��>�� B����>��>�� C����>��>�� D����>��>��
��3���ò��缫���һ��Ũ�ȵ��������ʵ�ˮ��Һ���ڵ������Һ�м�����ˮ����ʹ��ҺŨ�Ȼָ������ǰŨ�ȵ���___________��
A��NaOH B��CuSO4C��K2S D��NaCl
��4���Ƚ����(ѡ�>"�� <"��"=����
��2H2(g)+O2(g)= 2H2O(g)��H1��2H2(g)+O2(g)= 2H2O(l)��H2�ġ�H��С����H1 __________��H2��
�ڵ������PH����Һ��a������ b�����ᣬ�քe������NaOH��Һ��Ӧ������NaOH�����ʵ�
�����٣�a___________b��
�۳�����������Һ��a��pH=4���� b��pH=4NH4Cl��Һ������ˮ�ĵ���̶ȴ�С��a__________b��
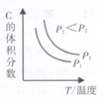
����֪ij���淴ӦaA(g)+bB(g)![]() cC(g)+dD(g)����������������ʱ��C������������¶ȣ�T����ѹǿ��P���Ĺ�ϵ����ͼ��ʾ����Ӧ���������Ļ�ѧ������֮�ͣ�a+b ______c+d��
cC(g)+dD(g)����������������ʱ��C������������¶ȣ�T����ѹǿ��P���Ĺ�ϵ����ͼ��ʾ����Ӧ���������Ļ�ѧ������֮�ͣ�a+b ______c+d��
��5����һ��������ܱ������м���1molCO2��1mol H2���������»�ѧ��Ӧ��CO2(g)+ H2(g) ![]() CO(g)+H2O(g) ���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
CO(g)+H2O(g) ���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
T(��) | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
�ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=__________________________��
�ڷ�ӦΪ___________��Ӧ������ȡ����ȡ�����
��ij�¶��£�ƽ��ʱ�����ʵ�Ũ�������¹�ϵ��3[c(CO2)c(H2)] =5[c(CO)(H2O)]���жϴ�ʱ�ķ�Ӧ�¶�Ϊ___________�档
��800�棬�������ڳ���lmolCO2��lmolH2��lmol CO��lmolH2O���˿̷�Ӧ��v��___________ v�������"������=����
��6����ͼ��ʾΪ�ö��Ե缫���100mL 0.5mol��CuSO4��Һ��װ�ã�b�缫�ϵĵ缫��ӦʽΪ______����a�缫������56mL����״�������壬��������Һ��pH =___________����������Һ����仯����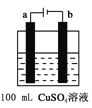
���𰸡� D B A > = < > ![]() ���� 700 < Cu2++2e-=Cu 1
���� 700 < Cu2++2e-=Cu 1
����������1��A��X��pHΪ1��С��7�������ԣ����������ǿ����������Һ��Ҳ��������ʽ����Һ��NaHSO4����A����B���¶����ߴٽ�ˮ�ĵ��룬90��ʱ�Ĵ�ˮpHС��7����B����C��û��ָ���¶ȣ�pH����7��Һ�岻һ�������ԣ���C����D��Z��pHΪ10������7���ʼ��ԣ�����������Һ�Լ��ԣ�����Z�����ǹ�������Һ����D��ȷ����ѡD��
��2����������������笠�����ˮ�⣬笠�����Ũ������е�������NH4+����笠�����ˮ��̶Ⱥ��������а�ˮ����̶Ⱥ�С��NH4+���ɵ�Ũ����С����c��NH4+����С˳��Ϊ���������ڣ���ѡB��
��3��A�����NaOH��Һʵ���ǵ��ˮ������������ʣ����Һ�м�����ˮ����ʹ��ҺŨ�Ⱥ͵��ǰ��ͬ����A��ȷ��B���������ͭʵ���ǵ������ͭ��ˮ������������ʣ����Һ�м�����ˮ������ʹ��ҺŨ�Ⱥ͵��ǰ��ͬ����B����C�����K2S��Һ������K2S��ˮ�����������ˮ���ָܻ�ԭ��Һ��Ũ�ȣ���C����D�����NaClʵ���ǵ��ˮ���Ȼ��ƣ�����������ʣ����Һ�м�����ˮ������ʹ��ҺŨ�Ⱥ͵��ǰ��ͬ����D����ѡA��
��4���Ƚ����(ѡ����>"���� <"��"=����
����̬ˮת��ΪҺ̬ˮ���ȣ�����ȫȼ������Һ̬ˮ�ų��������ߣ�����H1����H2��
�ڵ������PH�������������Һ��H+�����ʵ�����Ȳ���������NaOH��Һ��Ӧ����NaOH�����ʵ���Ҳ��ȣ���a=b��
�۳����£�pH��Ϊ4�������NH4Cl��Һ�У�Ӱ��ˮ������������������ˮ�ĵ��룬ˮ����δٽ�ˮ�ĵ��룬ˮ�ĵ���̶ȴ�С��ϵ��a��b��
����ͼʾ��֪����ͬ�¶��£�����ѹǿ��C�������������˵��ƽ�������ƶ����������������������С�ķ���a+b��c+d��
��5������ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮��������K=![]() ��
��
�ڻ�ѧƽ�ⳣ���Ĵ�Сֻ���¶��йأ������¶ȣ�ƽ�������ȵķ����ƶ����ɱ���֪�������¶ȣ���ѧƽ�ⳣ������˵����ѧƽ�������ƶ����������Ӧ�������ȣ�
��ƽ��Ũ�ȷ�����ʽ3[c(CO2)c(H2)] =5[c(CO)(H2O)]ʱ��K=![]() =0.6��ƽ�ⳣ��ֵֻ���¶ȵ�Ӱ�죬��K=0.6ʱ�����ݱ������ݣ������¶���700����
=0.6��ƽ�ⳣ��ֵֻ���¶ȵ�Ӱ�죬��K=0.6ʱ�����ݱ������ݣ������¶���700����
��800�棬�������ڳ���lmolCO2��lmolH2��lmol CO��lmolH2O����ʱQc=![]() =1��0.9����Ӧ������У��˿̷�Ӧ��v����v����
=1��0.9����Ӧ������У��˿̷�Ӧ��v����v����
��6����ͼ����ʾ���100mL0.5molL-1CuSO4��Һ�������ĵ��ط�ӦΪ��2CuSO4+2H2O![]() 2Cu+O2��+2H2SO4�����Դ����������Ϊ��������Һ��ͭ���ӵõ��ӷ�����ԭ��Ӧ����b�缫��ӦΪCu2++2e-=Cu��
2Cu+O2��+2H2SO4�����Դ����������Ϊ��������Һ��ͭ���ӵõ��ӷ�����ԭ��Ӧ����b�缫��ӦΪCu2++2e-=Cu��
��a�缫����56mL����״��������Ϊ���������ʵ���Ϊ0.0025mol�������������������ʵ���Ϊ0.01mol����Һ���������������ʵ���Ϊ0.01mol��c��H+��=![]() =0.1mol/L��pH=-lg0.1=1��
=0.1mol/L��pH=-lg0.1=1��

