��Ŀ����
��18�֣�
��. ����һ����Ҫ�Ļ���ԭ�ϣ�ijѧϰС������ȡ������̽�������ʡ�
��ش�
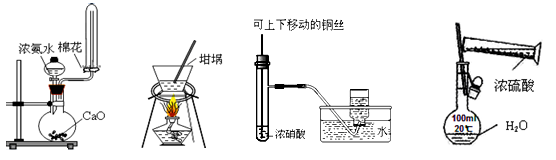
��1��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
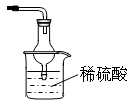
��2������ͼ�ǽ��а�����Ȫʵ���װ�ã�������Ȫ�IJ��������� ��

�ڰ���ʹ�ձ�����Һ����ɫ��Ϊ��ɫ����ԭ���ǣ��õ��뷽��ʽ��ʾ�� ��
��3����С��ͬѧ�������ͼ��ʾ��ʵ��װ�ã����ּг�����δ��������̽�������Ļ�ԭ�Բ�������

��ʵ������Ϊ����ɫCuO��Ϊ��ɫ����ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
�ڼ�ʯ�ҵ������� ��
������װ���ڷ���Ӧ����һ��װ�ú�ʵ����ƲŽ����ƣ����ڷ����в�����Eװ��ͼ(Ҫ��ע��װ���������Լ�����)��

��.3.2g Cu��30mL��8mol/L����HNO3��Ӧ������Ļ�ԭ����ΪNO��NO2����Ӧ����Һ������H+Ϊa mol���ٴ�ʱ��Һ��������NO3-Ϊ mol��
�������ɵ�NO�ڱ�״�������Ϊ L��(���Ͻ�����ú�a�Ĵ���ʽ��ʾ)
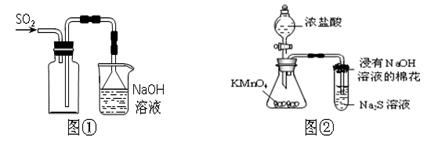
��.ij��ɫ������ܺ�������ͭ����ͭ������ͭ���Լ����ڱε�������ͭ��
��������֪������������ͭ�����Ի����»ᷢ������������ԭ��Ӧ����Cu2+��ͭ���ʣ��������������գ�����ת��Ϊ����ͭ������ͭ������ͭ�����¶�������ϡ���ᡣ�������������գ���ͭ������ͭ��ת��Ϊ����ͭ�Ͷ�������Ϊ���о��ijɷ֣���С��ͬѧ���ռ����㹻���Ĺ����������ͼ��ʾ��ʵ�飺

��1�����������չ�����һ�������ķ�Ӧ�Ļ�ѧ����ʽΪ ��
��2�����ڹ���ijɷֵ��ж��У�����˵����ȷ����
��. ����һ����Ҫ�Ļ���ԭ�ϣ�ijѧϰС������ȡ������̽�������ʡ�
��ش�
��1��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
��2������ͼ�ǽ��а�����Ȫʵ���װ�ã�������Ȫ�IJ��������� ��

�ڰ���ʹ�ձ�����Һ����ɫ��Ϊ��ɫ����ԭ���ǣ��õ��뷽��ʽ��ʾ�� ��
��3����С��ͬѧ�������ͼ��ʾ��ʵ��װ�ã����ּг�����δ��������̽�������Ļ�ԭ�Բ�������

��ʵ������Ϊ����ɫCuO��Ϊ��ɫ����ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
�ڼ�ʯ�ҵ������� ��
������װ���ڷ���Ӧ����һ��װ�ú�ʵ����ƲŽ����ƣ����ڷ����в�����Eװ��ͼ(Ҫ��ע��װ���������Լ�����)��

��.3.2g Cu��30mL��8mol/L����HNO3��Ӧ������Ļ�ԭ����ΪNO��NO2����Ӧ����Һ������H+Ϊa mol���ٴ�ʱ��Һ��������NO3-Ϊ mol��
�������ɵ�NO�ڱ�״�������Ϊ L��(���Ͻ�����ú�a�Ĵ���ʽ��ʾ)
��.ij��ɫ������ܺ�������ͭ����ͭ������ͭ���Լ����ڱε�������ͭ��
��������֪������������ͭ�����Ի����»ᷢ������������ԭ��Ӧ����Cu2+��ͭ���ʣ��������������գ�����ת��Ϊ����ͭ������ͭ������ͭ�����¶�������ϡ���ᡣ�������������գ���ͭ������ͭ��ת��Ϊ����ͭ�Ͷ�������Ϊ���о��ijɷ֣���С��ͬѧ���ռ����㹻���Ĺ����������ͼ��ʾ��ʵ�飺

��1�����������չ�����һ�������ķ�Ӧ�Ļ�ѧ����ʽΪ ��
��2�����ڹ���ijɷֵ��ж��У�����˵����ȷ����
| A��������У�CuS��Cu2S����ͬʱ���ڣ� |
| B��������У�CuO��Cu2O������һ�֣� |
| C�����������û��Cu2O����һ����Cu2S�� |
| D���������������Cu2O��Ҳ������Cu2S�� |
��.��1��2NH4Cl + Ca(OH)2 CaCl2 + 2NH3��+2H2O
CaCl2 + 2NH3��+2H2O
��2�������ἷѹ�ιܣ�ʹ����ˮ������ƿ��Ȼ���ֹˮ��K��
��NH3 + H2O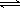 NH3��H2O
NH3��H2O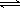 NH4+ + OH��
NH4+ + OH��
��3���� 3CuO + 2NH3 3Cu + N2 + 3H2O
3Cu + N2 + 3H2O
�����հ������е�ˮ��������ֹ���Ų���ˮ�IJⶨ��
����ͼ��ʾ�������������֣���
������²�CCl4�ϲ�H2O������ͨ��CCl4��
��. �٣�a+0.1��mol �� 11.2(a��0.04) L
��1��CuS+3O2 2SO2+2CuO �����������»����ն��ɣ�
2SO2+2CuO �����������»����ն��ɣ�
��2�� BCD
 CaCl2 + 2NH3��+2H2O
CaCl2 + 2NH3��+2H2O ��2�������ἷѹ�ιܣ�ʹ����ˮ������ƿ��Ȼ���ֹˮ��K��
��NH3 + H2O
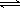 NH3��H2O
NH3��H2O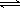 NH4+ + OH��
NH4+ + OH�� ��3���� 3CuO + 2NH3
 3Cu + N2 + 3H2O
3Cu + N2 + 3H2O �����հ������е�ˮ��������ֹ���Ų���ˮ�IJⶨ��
����ͼ��ʾ�������������֣���

������²�CCl4�ϲ�H2O������ͨ��CCl4��
��. �٣�a+0.1��mol �� 11.2(a��0.04) L
��1��CuS+3O2
 2SO2+2CuO �����������»����ն��ɣ�
2SO2+2CuO �����������»����ն��ɣ���2�� BCD
���������I. ��1��ʵ�����ü����Ȼ�狀��������ƵĻ������ȡ��������Ӧ����ʽΪ2NH4Cl + Ca(OH)2
 CaCl2 + 2NH3��+2H2O��
CaCl2 + 2NH3��+2H2O����2����Ҫ������Ȫ����ʹ��ƿ��ѹǿ��С����ѹ��ͷ�ιܣ�ʹ����ˮ������ƿ��������������ˮ��ʹ��ƿ��ѹǿ��С��Ȼ���ֹˮ��K�����ɿ�����Ȫ����
�ڰ�������ˮ�γɰ�ˮ����ˮ�Լ��ԣ���ʹ��̪��Һ��죬ԭ��ΪNH3 + H2O
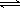 NH3��H2O
NH3��H2O 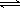 NH4+ + OH����
NH4+ + OH������3���ٺ�ɫ����ͭ��Ϊ��ɫ����ɫ����ΪCu����ɫ��ˮ����ͭ��Ϊ��ɫ��˵��������H2O����Ӧ��CuO����������NH3����ԭ����N���ϼ����ߣ��������ɵ���ɫ����ΪN2����Ӧ����ʽΪ3CuO + 2NH3
 3Cu + N2 + 3H2O��
3Cu + N2 + 3H2O���ڼ�ʯ�������Ǹ��ﰱ������ֹ����ˮ�ļ��顣
�۱�ʵ��β���к���NH3��NH3�ŷŵ������л���Ⱦ����������Ӧ�����ȥ�����ǵ�NH3��ˮ���ܽ�Ⱥܴ����Բ��ܰѰ���ֱ��ͨ��ˮ�У�Ӧ���÷�����װ�á�
II. ��Cu�����ϡ���ᷴӦ����Һ������������ͭ���������ᣬ���ݵ���غ�n(H+)+2n(Cu2+)=n(NO3��)������n(Cu2+)=n(Cu)=0.05mol��n(NO3��)=(a+0.1)mol��
��������NO��NO2���ʵ����ֱ�Ϊxmol��ymol���ɵ�ʧ������ȵã�3x+y=2n(Cu)����N�غ�ã�x+y+(a+0.1)mol=0.03��8mol�����x=(0.5a��0.02)mol
III���������ϡ����õ���ɫ��Һ��˵������ijɷ���CuO��Cu2O������һ�֡���������ΪCu��CuS��Cu2S�����췴Ӧ����ʽ��2Cu + O2
 2 CuO�������������ӣ���Cu2S+2O2
2 CuO�������������ӣ���Cu2S+2O2 2CuO+2SO2 �������������䣩��2CuS+3O2
2CuO+2SO2 �������������䣩��2CuS+3O2 2CuO+2SO2������������С�������й��������ɹ����������С��˵����������һ����CuS�����������չ�����һ�������ķ�Ӧ�Ļ�ѧ����ʽΪ2CuS+3O2
2CuO+2SO2������������С�������й��������ɹ����������С��˵����������һ����CuS�����������չ�����һ�������ķ�Ӧ�Ļ�ѧ����ʽΪ2CuS+3O2 2CuO+2SO2 ������2g��ȫ��CuS��������CuO1.67g�������й�����Ϊ1.84g��˵���������г���CuS�������������ʡ�
2CuO+2SO2 ������2g��ȫ��CuS��������CuO1.67g�������й�����Ϊ1.84g��˵���������г���CuS�������������ʡ������ҿ��ܵ�����У���CuS+CuO����CuS+Cu2S����CuS+CuO+Cu2S��
���Թ������һ����CuS�������������Cu2O��Cu2S���п��ޣ�����Cu2O����һ����Cu2S��
�����Ϸ�����֪�����ڹ���ijɷֵ��жϣ���ȷ����B��C��D��
������������չ�˽̲��еĵ���ʵ�飬���ػ���ʵ������������顣��ѧ����ע�ؽ��⼼�ɣ����������һ�⿼��˼ά������Ժ�����ԡ�

��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
�����Ŀ