ƒøƒ⁄»ð
°æƒø°ø£®1£©Õ¨Œ¬Õ¨—πœ¬£¨H2(g)£´Cl2(g)=2HCl(g)£¨‘⁄π‚’’∫Õµ„»ºÃıº˛œ¬µƒ¶§H(ªØ—ߺ∆¡ø ˝œýÕ¨)∑÷±Œ™¶§H1°¢¶§H2£¨¶§H1___¶§H2(ÃÓ°∞£æ°±°∞£º°±ªÚ°∞£Ω°±£¨œ¬Õ¨)°£
£®2£©œýÕ¨Ãıº˛œ¬£¨1 molP4À˘æþ”–µƒƒÐ¡ø___4 molP‘≠◊”À˘æþ”–µƒƒÐ¡ø°£
£®3£©“—÷™P4(∞◊¡◊£¨s)4P(∫Ï¡◊£¨s) ¶§H£Ω-17kJ°§mol£≠1£¨±»Ωœœ¬¡–∑¥”¶÷–¶§Hµƒ¥Û–°£∫¶§H1___¶§H2°£
¢ŸP4(∞◊¡◊£¨s)£´5O2(g)=2P2O5(s) ¶§H1
¢⁄4P(∫Ï¡◊£¨s)£´5O2(g)=2P2O5(s) ¶§H2
£®4£©“—÷™£∫œ°»Ð“∫÷–£¨H+£®aq£©+OH-£®aq£©=H2O£®l£© ¶§H=-57.3kJ°§mol-1£¨‘Ú≈®¡ÚÀ·∫Õœ°«‚—ıªØƒ∆»Ð“∫∑¥”¶…˙≥…2molÀÆ£¨∑≈≥ˆµƒ»»¡ø___114.6kJ°£
£®5£©“—÷™£∫28gFe(s)”ÎCO2(g)‘⁄“ª∂®Ãıº˛œ¬£¨ÕÍ»´∑¥”¶…˙≥…FeO(s)∫ÕCO(g)£¨Œ¸ ’¡ÀakJ»»¡ø£¨∏√∑¥”¶µƒ»»ªØ—ß∑Ω≥Ã Ω «___°£
°æ¥∞∏°ø= £º £º £æ Fe(s)+CO2(g)= FeO(s)+CO(g) ![]() H=+2akJ/mol
H=+2akJ/mol
°æΩ‚Œˆ°ø
£®1£©∑¥”¶»»”Î∑¥”¶ŒÔµƒ◊ЃСø∫Õ…˙≥…ŒÔµƒ◊ЃСø£¨”Î∑¥”¶Ãıº˛ŒÞπÿ£¨‘Úπ‚’’∫Õµ„»ºÃıº˛µƒ°˜HœýÕ¨£¨
π ¥∞∏Œ™£∫=£ª
£®2£©P‘≠◊”–Œ≥…P4∑÷◊” ±–Œ≥…ªØ—ߺ¸£¨ Õ∑≈ƒÐ¡ø£¨π 1 molP4À˘æþ”–µƒƒÐ¡ø£º4 molP‘≠◊”À˘æþ”–µƒƒÐ¡ø£ª
π ¥∞∏Œ™£∫£º£ª
£®3£©∏˘æðÂ∏¯»»ªØ—ß∑Ω≥Ã Ω£¨≥£Œ¬ ±∫Ï¡◊±»∞◊¡◊Œ»∂®£¨Àµ√˜∞◊¡◊ƒÐ¡ø∏þ£¨∑¥”¶∑≈≥ˆµƒ»»¡øΩœ∂ý£¨“Ú°˜H£º0£¨‘Ú∑≈≥ˆµƒƒÐ¡ø‘Ω∂ý°˜H‘Ω–°£¨
π ¥∞∏Œ™£∫£º£ª
£®4£©≈®¡ÚÀ·»Ð”⁄ÀÆ∑≈»»£¨π ≈®¡ÚÀ·∫Õœ°«‚—ıªØƒ∆»Ð“∫∑¥”¶…˙≥…2molÀÆ£¨∑≈≥ˆµƒ»»¡ø£æ114.6kJ£¨
π ¥∞∏Œ™£∫£æ£ª
£®5£©“—÷™£∫28gFe(s)º¥0.5mol Fe(s)”ÎCO2(g)‘⁄“ª∂®Ãıº˛œ¬£¨ÕÍ»´∑¥”¶…˙≥…FeO(s)∫ÕCO(g)£¨Œ¸ ’¡ÀakJ»»¡ø£¨∏√∑¥”¶µƒ»»ªØ—ß∑Ω≥Ã Ω «Fe(s)+CO2(g)= FeO(s)+CO(g) ![]() H=+2akJ/mol°£
H=+2akJ/mol°£

 ÃÏÃϜڅœ“ª±æ∫√æÌœµ¡–¥∞∏
ÃÏÃϜڅœ“ª±æ∫√æÌœµ¡–¥∞∏ –°—ß…˙10∑÷÷””¶”√Âœµ¡–¥∞∏
–°—ß…˙10∑÷÷””¶”√Âœµ¡–¥∞∏°æƒø°ø µ—È–°◊È∂‘NaHSO3»Ð“∫∑÷±”ÎCuCl2°¢CuSO4»Ð“∫µƒ∑¥”¶Ω¯––ÃΩæø°£
µ—È | ◊∞÷√ | ‘º¡x | ≤Ÿ◊˜º∞œ÷œÛ |
¢Ò |
| 1 mol°§L1 CuCl2»Ð“∫ | º”»Î2mL CuCl2»Ð“∫£¨µ√µΩ¬Ã…´»Ð“∫£¨30s ±”–ŒÞ…´∆¯≈ð∫Õ∞◊…´≥¡µÌ≤˙…˙£¨…œ≤„»Ð“∫—’…´±‰«≥°£ |
¢Ú | 1 mol°§L1 CuSO4»Ð“∫ | º”»Î2mL CuSO4»Ð“∫£¨µ√µΩ¬Ã…´»Ð“∫£¨3∑÷÷”Œ¥º˚√˜œ‘±‰ªØ°£ |
“—÷™£∫¢Ò.Cu2+![]() [Cu(NH3)4]2+(…Ó¿∂…´»Ð“∫)
[Cu(NH3)4]2+(…Ó¿∂…´»Ð“∫)
¢Ú. Cu+![]() [Cu(NH3)]+(ŒÞ…´»Ð“∫)
[Cu(NH3)]+(ŒÞ…´»Ð“∫)![]() [Cu(NH3)4]2+(…Ó¿∂…´»Ð“∫)
[Cu(NH3)4]2+(…Ó¿∂…´»Ð“∫)
(1)Õ∆≤‚ µ—È¢Ò≤˙…˙µƒŒÞ…´∆¯ÃÂŒ™SO2£¨ µ—È÷§ µÕ∆≤‚’˝»∑£∫”√’∫”–µ‚ÀƵƒµÌ∑€ ‘÷ΩΩ”Ω¸ ‘πÐø⁄£¨π€≤ϵΩ_______£¨∑¥”¶µƒ¿Î◊”∑Ω≥Ã ΩŒ™_______°£
(2)∂‘ µ—È ¢Ò ≤˙…˙SO2µƒ‘≠“ÚΩ¯––∑÷Œˆ£¨Ã·≥ˆºŸ…Ë£∫
ºŸ…Ëa: Cu2+ÀÆΩ‚ π»Ð“∫÷–c(H+)‘ˆ¥Û£ª
ºŸ…Ëb: Cl£≠¥Ê‘⁄ ±£¨Cu2+”ÎHSO3£≠∑¥”¶…˙≥…CuCl∞◊…´≥¡µÌ£¨»Ð“∫÷–c(H+)‘ˆ¥Û°£
¢Ÿ ºŸ…Ëa≤ª∫œ¿Ì£¨ µ—È÷§æð «_______£ª
¢⁄ µ—ȱÌ√˜ºŸ…Ëb∫œ¿Ì£¨ µ—ÈI∑¥”¶µƒ¿Î◊”∑Ω≥Ã Ω”–_____°¢H+ + HSO3- = SO2°¸+H2O°£
(3)∂‘±» µ—È¢Ò°¢¢Ú£¨Ã·≥ˆºŸ…Ë£∫Cl£≠‘ˆ«ø¡ÀCu2+µƒ—ıªØ–‘°£
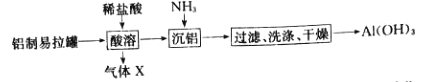
œ¬ ˆ µ—È¢Û÷§ µ¡ÀºŸ…Ë∫œ¿Ì£¨◊∞÷√»ÁÕº°£ µ—È∑Ω∞∏£∫±’∫œK£¨µÁ—π±Ìµƒ÷∏’Î∆´◊™÷¡°∞X°±¥¶£ªœÚU–ŒπÐ_______(≤π»´ µ—È≤Ÿ◊˜º∞œ÷œÛ)°£

(4)Ω´ µ—È¢Úµƒ»Ð“∫æ≤÷√24–° ±ªÚº”»»∫Û£¨µ√µΩ∫Ï…´≥¡µÌ°£æ≠ºÏ—È£¨∫Ï…´≥¡µÌ÷–∫¨”–Cu+°¢Cu2+∫ÕSO32°£
¢ŸÕ®π˝ µ—È¢Ù÷§ µ∫Ï…´≥¡µÌ÷–∫¨”–Cu+∫ÕCu2+°£
µ—È¢Ù£∫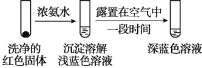
÷§ µ∫Ï…´≥¡µÌ÷–∫¨”–Cu+µƒ µ—È÷§æð «_______£ª
¢⁄”–Õ¨—ß»œŒ™ µ—È¢Ù≤ª◊„“‘÷§ µ∫Ï…´≥¡µÌ÷–∫¨”–Cu2+£¨…˺∆ µ—È¢Ùµƒ∂‘±» µ—È¢ı£¨÷§ µ¡ÀCu2+µƒ¥Ê‘⁄°£ µ—È¢ıµƒ∑Ω∞∏∫Õœ÷œÛ «£∫_______°£
°æƒø°ø¢≈ø…ƒÊ∑¥”¶FeO£®s£©+CO£®g£©Fe£®s£©+CO2£®g£© «¡∂Ã˙𧓵÷–“ª∏ˆ÷ÿ“™∑¥”¶£¨∆‰Œ¬∂»”Î∆Ω∫‚≥£ ˝Kµƒπÿœµ»Áœ¬±Ì£∫
T/K | 938 | 1100 |
K | 0.68 | 0.40 |
¢Ÿ–¥≥ˆ∏√∑¥”¶∆Ω∫‚≥£ ˝µƒ±Ì¥Ô Ω________°£
¢⁄»Ù∏√∑¥”¶‘⁄ê˝πÃ∂®µƒ√б’»ð∆˜÷–Ω¯––£¨‘⁄“ª∂®Ãıº˛œ¬¥ÔµΩ∆Ω∫‚◊¥Ã¨£¨»Ù…˝∏þŒ¬∂»£¨ªÏ∫œ∆¯Ãµƒ∆Ωæ˘œý∂‘∑÷◊”÷ ¡ø________£ª≥‰»Î∫§∆¯£¨ªÏ∫œ∆¯Ãµƒ√Ð∂»________£®ÃÓ°∞‘ˆ¥Û°±°∞ºı–°°±ªÚ°∞≤ª±‰°±£©°£
¢∆≥£Œ¬œ¬£¨≈®∂»æ˘Œ™0.1 mol°§L-1µƒœ¬¡–¡˘÷÷»Ð“∫µƒpH»Áœ¬±Ì£∫
»Ð÷ | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
¢Ÿ∏˘æð±Ì÷– ˝æð≈–∂œ£¨≈®∂»æ˘Œ™0.01 mol°§L-1µƒœ¬¡–Àƒ÷÷ŒÔ÷ µƒ»Ð“∫÷–£¨À·–‘◊Ó«øµƒ «________ £®ÃÓ±ý∫≈£©°£
A£ÆHCN B£ÆHClO C£ÆH2CO3 D£ÆCH3COOH
¢⁄æð…œ±Ì ˝æ𣨫΃„≈–∂œœ¬¡–∑¥”¶≤ªƒÐ≥…¡¢µƒ «________£®ÃÓ±ý∫≈£©°£
A£ÆCH3COOH+Na2CO3=NaHCO3+CH3COONa B£ÆCH3COOH+NaCN=CH3COONa+HCN C£ÆCO2+H2O+2NaClO=Na2CO3+2HClO
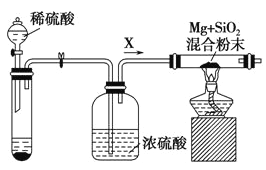
°æƒø°øœ¬¡– µ—È÷–£¨ƒÐ¥ÔµΩœý”¶ µ—ȃøµƒµƒ «
|
|
|
|
A£Æ÷∆±∏≤¢ ’ºØ““À·““ı• | B£Æ÷§√˜¬»ªØ“¯»ÐΩ‚∂»¥Û”⁄¡ÚªØ“¯ | C£Æ—È÷§‰Â““Õȵƒœ˚»•≤˙ŒÔ «““œ© | D£ÆÕ∆∂œS°¢C°¢Siµƒ∑«Ω Ù–‘«ø»ı |
A.AB.BC.CD.D