��Ŀ����
����Ŀ��ʵ��С���NaHSO3��Һ�ֱ���CuCl2��CuSO4��Һ�ķ�Ӧ����̽����
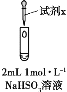
ʵ�� | װ�� | �Լ�x | ���������� |
�� |
| 1 mol��L1 CuCl2��Һ | ����2mL CuCl2��Һ���õ���ɫ��Һ��30sʱ����ɫ���ݺͰ�ɫ�����������ϲ���Һ��ɫ��dz�� |
�� | 1 mol��L1 CuSO4��Һ | ����2mL CuSO4��Һ���õ���ɫ��Һ��3����δ�����Ա仯�� |
��֪����.Cu2+![]() [Cu(NH3)4]2+(����ɫ��Һ)
[Cu(NH3)4]2+(����ɫ��Һ)
��. Cu+![]() [Cu(NH3)]+(��ɫ��Һ)
[Cu(NH3)]+(��ɫ��Һ)![]() [Cu(NH3)4]2+(����ɫ��Һ)
[Cu(NH3)4]2+(����ɫ��Һ)
(1)�Ʋ�ʵ����������ɫ����ΪSO2��ʵ��֤ʵ�Ʋ���ȷ����պ�е�ˮ�ĵ�����ֽ�ӽ��Թܿڣ��۲쵽_______����Ӧ�����ӷ���ʽΪ_______��
(2)��ʵ�� �� ����SO2��ԭ����з�����������裺
����a: Cu2+ˮ��ʹ��Һ��c(H+)����
����b: Cl������ʱ��Cu2+��HSO3����Ӧ����CuCl��ɫ��������Һ��c(H+)����
�� ����a��������ʵ��֤����_______��
�� ʵ���������b������ʵ��I��Ӧ�����ӷ���ʽ��_____��H+ + HSO3- = SO2��+H2O��
(3)�Ա�ʵ���������裺Cl����ǿ��Cu2+�������ԡ�
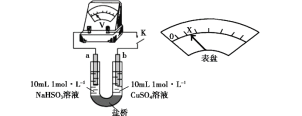
����ʵ���֤ʵ�˼��������װ����ͼ��ʵ�鷽�����պ�K����ѹ����ָ��ƫת����X��������U�ι�_______(��ȫʵ�����������)��
(4)��ʵ������Һ����24Сʱ����Ⱥõ���ɫ�����������飬��ɫ�����к���Cu+��Cu2+��SO32��
��ͨ��ʵ���֤ʵ��ɫ�����к���Cu+��Cu2+��
ʵ�����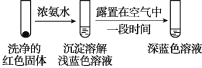
֤ʵ��ɫ�����к���Cu+��ʵ��֤����_______��
����ͬѧ��Ϊʵ���������֤ʵ��ɫ�����к���Cu2+�����ʵ����ĶԱ�ʵ�����֤ʵ��Cu2+�Ĵ��ڡ�ʵ����ķ����������ǣ�_______��
���𰸡���ɫ��ȥ SO2+I2+2H2O=SO42��+2I��+4H+ ʵ�����c(Cu2+)��ͬ����ʵ��� ��δ������ 2Cu2++2Cl��+ HSO3��+ H2O =2CuCl��+SO42-+3H+ �Ҳ����һ����NaCl���壬�ܽ�۲쵽��ѹ��ָ��ƫת��� һ��ʱ�����Һ��dz��ɫ��Ϊ����ɫ ȡ����������Cu2O���Թ��У��μ�����Ũ��ˮ�������ܽ⣬�õ���ɫ��Һ��¶��һ��ʱ�����Һ��Ϊ����ɫ
��������
NaHSO3��Һ�����������ʽ�Σ�HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�������Һ�����ԡ�
(1) �Ӷ�������Ļ�ԭ�Ժ͵�������ԽǶȳ�����д��ʵ����������ӷ�Ӧ����ʽ��
(2) Ҫ���������������壬��Ҫ��Һ���������Ӵ��ڣ����������Ӳ�����ԭ���Դ�ʵ�����ͭ���ӣ�������ͬ���ʿ����жϳ�����a����ȷ��
(3) �Ƿ���Cl����ǿ��Cu2+�������ԣ����Լ��������ӣ�����Ϊ�˲������µ����ʣ����Լ��Ȼ��ƣ��ٹ۲��ѹ����ʾ����
(4)������Ŀ��Ϣ�� Cu+![]() [Cu(NH3)]+(��ɫ��Һ)
[Cu(NH3)]+(��ɫ��Һ)![]() [Cu(NH3)4]2+(����ɫ��Һ)��ȷ��Cu+�ļ��鷽������Ҫ֤����Һ�е�Cu2+����Ҫ��һ���Ա�ʵ�飬һ������Cu2+��Cu+�Ļ����Һ����һ��ֻ����Cu+����Һ���ټ�������Ũ��ˮ������Ӧ�������Կ��Լ�����������Cu2O��
[Cu(NH3)4]2+(����ɫ��Һ)��ȷ��Cu+�ļ��鷽������Ҫ֤����Һ�е�Cu2+����Ҫ��һ���Ա�ʵ�飬һ������Cu2+��Cu+�Ļ����Һ����һ��ֻ����Cu+����Һ���ټ�������Ũ��ˮ������Ӧ�������Կ��Լ�����������Cu2O��
(1)պ�е�ˮ�ĵ�����ֽ����ɫ����������������������л�ԭ�ԣ��ⵥ�ʾ��������ԣ�����������ⵥ�ʷ���������ԭ��Ӧ��ʵ������Ϊ��ɫ��ȥ�����ӷ���ʽΪSO2+I2+ 2H2O=SO42��+2I��+4H+��
�ʴ�Ϊ����ɫ��ȥ��SO2+I2+2H2O=SO42��+2I��+4H+��
(2)Ҫ��������������Ҫ������������������������ã������Cu2+ˮ��ʹ��Һ��������Ũ�����Ӷ��ͷų����壬����ʵ����������c(Cu2+)��ͬ����ʵ���� ��δ�����ݣ�˵�������ɼ���a: Cu2+ˮ��ʹ��Һ��c(H+)��������ģ��ʴ�Ϊʵ����������c(Cu2+)��ͬ����ʵ������δ�����ݣ��������������Ƽ����Ȼ�ͭ���ʵ�����������ɰ�ɫ�����������b��ȷ�����õ���غ㣬Ԫ���غ㣬���ϼ���������ƽ���ӷ�Ӧ��2Cu2++2Cl��+HSO3��+H2O =2CuCl��+SO42-+3H+��
�ʴ�Ϊ��ʵ����������c(Cu2+)��ͬ����ʵ���� ��δ�����ݣ�2Cu2++2Cl��+ HSO3��+ H2O =2CuCl��+SO42-+3H+��
(3) ʵ������Ϊ����֤Cl����ǿ��Cu2+�������ԣ���������ͭ��Һ�в��������ӣ�U����������������ƣ��ʼ����Ȼ��ƹ��壬���Cl����ǿ��Cu2+�������ԣ���ʹ��Һ��ת�Ƶĵ��������࣬�պ�K���ʵ�ѹ���Ķ���������
�ʴ�Ϊ���Ҳ����һ����NaCl���壬�ܽ�۲쵽��ѹ��ָ��ƫת���
(4) ������Ŀ������Ϣ��Cu+![]() [Cu(NH3)]+(��ɫ��Һ)
[Cu(NH3)]+(��ɫ��Һ)![]() [Cu(NH3)4]2+(����ɫ��Һ)��ʵ���������Cu+��һ��ʱ�����Һ��dz��ɫ��Ϊ����ɫ����Ҫ֤����Һ�е�Cu2+����Ҫ��һ���Ա�ʵ�飬һ������Cu2+��Cu+�Ļ����Һ����һ��ֻ����Cu+����Һ���ټ�������Ũ��ˮ�������ܽ⣬�õ���ɫ��Һ��¶��һ��ʱ�����Һ��Ϊ����ɫ��ʵ�����Ϊȡ����������Cu2O���Թ��У��μ�����Ũ��ˮ��ʵ������Ϊ�����ܽ⣬�õ���ɫ��Һ��¶��һ��ʱ�����Һ��Ϊ����ɫ��
[Cu(NH3)4]2+(����ɫ��Һ)��ʵ���������Cu+��һ��ʱ�����Һ��dz��ɫ��Ϊ����ɫ����Ҫ֤����Һ�е�Cu2+����Ҫ��һ���Ա�ʵ�飬һ������Cu2+��Cu+�Ļ����Һ����һ��ֻ����Cu+����Һ���ټ�������Ũ��ˮ�������ܽ⣬�õ���ɫ��Һ��¶��һ��ʱ�����Һ��Ϊ����ɫ��ʵ�����Ϊȡ����������Cu2O���Թ��У��μ�����Ũ��ˮ��ʵ������Ϊ�����ܽ⣬�õ���ɫ��Һ��¶��һ��ʱ�����Һ��Ϊ����ɫ��
�ʴ�Ϊ��һ��ʱ�����Һ��dz��ɫ��Ϊ����ɫ��ȡ����������Cu2O���Թ��У��μ�����Ũ��ˮ�� �����ܽ⣬�õ���ɫ��Һ��¶��һ��ʱ�����Һ��Ϊ����ɫ��

����Ŀ�����¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ�������ӦN2(g)+3H2(g)![]() 2NH3(g) ��H= 92.4kJ/mol�����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
2NH3(g) ��H= 92.4kJ/mol�����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
���� | �� | �� | �� |
��Ӧ��Ͷ���� | 0.5mol N2��1.5mol H2 | 1mol NH3 | 2mol NH3 |
NH3��Ũ��(mol/L) | c1 | c2 | c3 |
���ջ�ų�������(kJ) | a | b | c |
��ϵѹǿ(Pa) | p1 | p2 | p3 |
��Ӧ��ת���� | ��1 | ��2 | ��3 |
����˵����ȷ����
A.a + b = 46.2B.2c1��c3��c1C.2p2��p3D.��1+��3��1