ΧβΡΩΡΎ»ί
18Θ°»γ±μ÷–ΤάΦέΚœάμΒΡ «Θ®ΓΓΓΓΘ©| ―Γœν | Μ·―ßΖ¥”ΠΦΑΤδάκΉ”ΖΫ≥Χ Ϋ | ΤάΦέ |
| A | Fe3O4”κœΓœθΥαΖ¥”ΠΘΚ2Fe3O4+18H+®T6Fe3++H2Γϋ+ 8H2O | ’ΐ»Ζ |
| B | œρΧΦΥαΟΨ÷–Φ”œΓ―ΈΥαΘΚCO${\;}_{3}^{2-}$+2H+®TCO2Γϋ+ H2O | ¥μΈσΘ§ΧΦΥαΟΨ≤Μ”Π–¥≥…άκΉ”–Έ Ϋ |
| C | œρΝρΥαοß»ή“Κ÷–Φ”»κ«β―θΜ·±Β»ή“ΚΘΚBa2++SO${\;}_{4}^{2-}$®TBaSO4Γΐ | ’ΐ»Ζ |
| D | FeBr2»ή“Κ”κΒ»Έο÷ ΒΡΝΩΒΡCl2Ζ¥”ΠΘΚ2Fe2++2Br-+2Cl2®T2Fe3++4Cl-+ Br2 | ¥μΈσΘ§Fe2+”κBr-ΒΡΜ·―ßΦΤΝΩ ΐ÷°±»”ΠΈΣ1ΘΚ2 |
| AΘ° | A | BΘ° | B | CΘ° | C | DΘ° | D |
Ζ÷Έω AΘ°œθΥα”κΥΡ―θΜ·»ΐΧζΖΔ…ζ―θΜ·ΜΙ‘≠Ζ¥”Π…ζ≥…œθΥαΧζΓΔ“Μ―θΜ·ΒΣΚΆΥ°ΘΜ
BΘ°ΧΦΥαΟΨΈΣ≥ΝΒμΘ§”Π±ΘΝτΜ·―ß ΫΘΜ
CΘ°¬©ΒτοßΗυάκΉ””κ«β―θΗυάκΉ”ΒΡΖ¥”ΠΘΜ
DΘ°―«ΧζάκΉ”ΒΡΜΙ‘≠–‘«Ω”ΎδεάκΉ”Θ§¬»Τχœ»―θΜ·ΕΰΦέΧζάκΉ”Θ°
Ϋβ¥π ΫβΘΚAΘ°Fe3O4”κœΓœθΥαΖ¥”Π≤ζ…ζΒΡΤχΧε «“Μ―θΜ·ΒΣΘ§≤ΜΜα≤ζ…ζ«βΤχΘ§’ΐ»ΖΒΡάκΉ”ΖΫ≥Χ ΫΘΚ3Fe3O4 +28H++NO3-=9Fe3++NOΓϋ+14H2OΘ§Ι A¥μΈσΘΜ
BΘ°œρΧΦΥαΟΨ÷–Φ”œΓ―ΈΥαΘ§άκΉ”ΖΫ≥Χ ΫΘΚMgCO3+2H+®TMg2++CO2Γϋ+H2OΘ§Ι B’ΐ»ΖΘΜ
CΘ°οßΗυάκΉ”ΡήΚΆ«β―θΗυάκΉ”Ζ¥”Π≤ζ…ζΑ±ΤχΜρ’Ώ «“ΜΥ°ΚœΑ±Θ§œρΝρΥαοß»ή“Κ÷–Φ”»κ«β―θΜ·±Β»ή“ΚΘ§Ζ¥”ΠΈΣΘΚ2NH4++Ba2++SO42-+2OH-®TBaSO4Γΐ+2NH3•H2OΘ§Ι C¥μΈσΘΜ
DΘ°―«ΧζάκΉ”ΒΡΜΙ‘≠–‘«Ω”ΎδεάκΉ”Θ§FeBr2»ή“Κ”κΒ»Έο÷ ΒΡΝΩΒΡCl2Ζ¥”ΠΈΣΘ§―«ΧζάκΉ”±ΜΆξ»Ϊ―θΜ·Θ§δεάκΉ”±Μ≤ΩΖ÷―θΜ·ΘΚ2Fe2++2Br-+2Cl2=2Fe3++4Cl-+Br2Θ§»τFe2+”κBr-ΒΡΜ·―ßΦΤΝΩ ΐ÷°±»”ΠΈΣ1ΘΚ2Θ§‘ρ¬»Τχ–η“ΣΙΐΝΩΘ§δεΜ·―«Χζ»Ϊ≤ΩΖ¥”ΠΘ§Ι D¥μΈσΘΜ
Ι ―ΓΘΚBΘ°
ΒψΤά ±ΨΧβΩΦ≤ιΝΥάκΉ”ΖΫ≥Χ ΫΒΡ ι–¥Θ§Ος»ΖΖ¥”Π Β÷ «ΫβΧβΙΊΦϋΘ§ΉΔ“βΖ¥”ΠΈο”ΟΝΩΕ‘Ζ¥”ΠΒΡ”ΑœλΘ§―ΓœνDΈΣ“Ή¥μ―ΓœνΘ°

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ| AΘ° | Na+ΓΔK+ΓΔCl-ΓΔSO42- | BΘ° | Na+ΓΔHCO3-ΓΔSO42-ΓΔCl- | ||
| CΘ° | Fe2+ΓΔK+ΓΔSO42-ΓΔNO3- | DΘ° | K+ΓΔNH4+ΓΔSO42-ΓΔCl- |
| AΘ° | ”ξΥ°―υΤΖΥα–‘÷πΫΞΦθ–Γ | |
| BΘ° | ”ξΥ°―υΤΖΥα–‘ΟΜ”–±δΜ· | |
| CΘ° | ”ξΥ°―υΤΖpHΫΒΒΆΒΡ‘≠“ρΩ…Ρή «ΦΧ–χΈϋ ’Ω’Τχ÷–ΒΡCO2 | |
| DΘ° | ”ξΥ°―υΤΖ÷–ΒΡpHΫΒΒΆΒΡ‘≠“ρΩ…Ρή «H2SO3÷πΫΞ±Μ―θΤχ―θΜ·≥…H2SO4 |
| AΘ° | 0.15 mol/L | BΘ° | 0.20 mol/L | CΘ° | 0.25 mol/L | DΘ° | 0.40 mol/L |
| AΘ° | ‘Ύ101kPa ±Θ§1mol C”κ ΝΩO2Ζ¥”Π…ζ≥…1mol CO ±Θ§Ζ≈≥ω110.5kJ»»ΝΩΘ§‘ρCΒΡ»Φ…’»»ΈΣ110.5kJ/mol | |
| BΘ° | ‘Ύ10lkPa ±lmol H2Άξ»Ϊ»Φ…’…ζ≥…“ΚΧ§Υ°Ζ≈≥ω285.8kJ»»ΝΩΘ§H2»Φ…’»»ΈΣ-285.8KJ/mol | |
| CΘ° | ΦχΕ®HClΚΆNaOHΖ¥”ΠΒΡ÷–ΚΆ»» ±Θ§ΟΩ¥Έ Β―ιΨυ”Π≤βΝΩ3ΗωΈ¬Ε»Φ¥―ΈΥαΤπ ΦΈ¬Ε»ΓΔNaOHΤπ ΦΈ¬Ε»ΚΆΖ¥”ΠΚσ÷’÷ΙΈ¬Ε» | |
| DΘ° | ‘ΎœΓ»ή“Κ÷–ΘΚH+Θ®aqΘ©+oH-Θ®aqΘ©®TH2OΘ®lΘ©ΓςH=-57.3KJ/molΘ§»τΫΪΚ§0.5molH2SO4ΒΡ≈®ΝρΥα”κΚ§1mol NaOHΒΡ»ή“ΚΜλΚœΘ§Ζ≈≥ωΒΡ»»ΝΩΒ»”Ύ57.3KJ |
| AΘ° | NaHCO3ΒΡΒγάκΘΚNaHCO3?Na++H++CO32- | |
| BΘ° | HS-ΒΡΥ°ΫβΘΚHS-+H2O?H3O++S2- | |
| CΘ° | ΦΉΆιΒΡ±ξΉΦ»Φ…’»»ΈΣ-890.3kJ•mol-1Θ§‘ρΦΉΆι»Φ…’ΒΡ»»Μ·―ßΖΫ≥Χ ΫΩ…±μ ΨΈΣΘΚCH4Θ®gΘ©+2O2Θ®gΘ©®TCO2Θ®gΘ©+2H2O Θ®gΘ©ΓςH=-890.3kJ•mol-1 | |
| DΘ° | ΥΪ―θΥ°÷–Φ”»κœΓΝρΥαΚΆKI»ή“ΚΘΚH2O2+2I-+2H+®TI2+2H2O |
 Ρ≥Έ¬Ε»Θ®TΓφΘ©œ¬ΒΡ»ή“Κ÷–Θ§cΘ®H+Θ©=10-x mol•L-1Θ§cΘ®OH-Θ©=10-y mol•L-1Θ§x”κyΒΡΙΊœΒ»γΆΦΥυ ΨΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Ρ≥Έ¬Ε»Θ®TΓφΘ©œ¬ΒΡ»ή“Κ÷–Θ§cΘ®H+Θ©=10-x mol•L-1Θ§cΘ®OH-Θ©=10-y mol•L-1Θ§x”κyΒΡΙΊœΒ»γΆΦΥυ ΨΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚΘ®1Θ©ΗΟΈ¬Ε»TΘΨ25ΓφΘ®ΧνΓΑΘΨΓ±ΓΔΓΑΘΦΓ±ΜρΓΑ=Γ±Θ©Θ°
Θ®2Θ©‘Ύ¥ΥΈ¬Ε»œ¬Θ§œρBaΘ®OHΘ©2»ή“Κ÷–÷πΒΈΦ”»κpH=aΒΡ―ΈΥαΘ§≤βΒΟΜλΚœ»ή“ΚΒΡ≤ΩΖ÷pH»γ±μΥυ ΨΘ°
| Β―ι –ρΚ≈ | BaΘ®OHΘ©2»ή“Κ ΒΡΧεΜΐ/mL | ―ΈΥαΒΡΧεΜΐ/mL | »ή“ΚΒΡpH |
| ΔΌ | 22.00 | 0.00 | 9 |
| ΔΎ | 22.00 | 18.00 | b |
| Δέ | 22.00 | 22.00 | 6 |
Θ®3Θ©‘Ύ¥ΥΈ¬Ε»œ¬Θ§ΫΪ10mL 0.1mol•L-1ΒΡBaΘ®OHΘ©2»ή“ΚΖ÷±π”κ5mL 0.1mol•L-1ΒΡNaHSO4»ή“ΚΜλΚœΘ§ΥυΒΟ»ή“ΚΒΡpHΈΣ12Θ°»γΙϊΫΪΒ»≈®Ε»Β»ΧεΜΐΒΡBaΘ®OHΘ©2»ή“Κ”κNH4Cl»ή“ΚΜλΚœΘ§ΥυΒΟ»ή“Κ÷–ΗςάκΉ”≈®Ε»”…¥σΒΫ–ΓΒΡ≈≈Ν–Υ≥–ρ «cΘ®OH-Θ©ΘΨcΘ®Ba2+Θ©=cΘ®Cl-Θ©ΘΨcΘ®NH4+Θ©ΘΨcΘ®H+Θ©Θ°
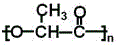 Θ°
Θ°