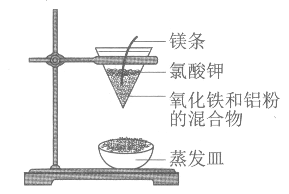
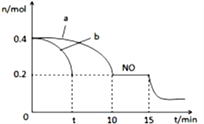
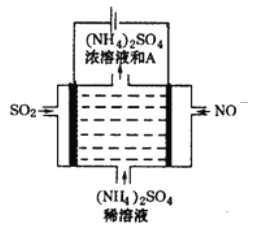
��Ŀ����
����Ŀ����Ԫ�ص��⻯����������ڹ�ҵ�������������ж��й㷺Ӧ�ã��ش��������⣺
(1)��Ԫ��ԭ�ӵ� L �������Ϊ ___________��
(2)��(N2H4)�ֳ���������ɫ��״Һ�塣�������ڰ��Ĵ̱���ζ������Ϊ�����������ȼ�ϡ�
��)���е�Ԫ�صĻ��ϼ�Ϊ ____��
��NH3 �� NaClO ��Ӧ�ɵõ��£��÷�Ӧ�Ļ�ѧ����ʽΪ_____��
��16g Һ̬���ڿ�����ȼ�գ����ɵ�����ˮ����ʱ�ų�������Ϊ 267.1kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ�� ____��
���𰸡�5 -2 2NH3+NaClO === N2H4 + NaCl + H2O N2H4(1)+ O2(g)=== N2(g)+ 2H2O(g),��H=-534.2kJmol-1
��������
(1)����ԭ�Ӻ�����ӷֲ��Ų��Ĺ��ɿ�ȷ����ԭ�ӵ�L���������
(2) ��������Ԫ����-1�ۣ����ݻ������и�Ԫ�ػ��ϼ۴�����Ϊ0��ȷ����Ԫ�صĻ��ϼۣ��ڸ���NaClO����ǿ��������NH3�е�Ԫ����-3�ۣ��л�ԭ�ԣ�����֪NaClO�ܽ�NH3���������ݵ�ʧ�����غ��ԭ���غ���ƽ������ȷ��1molN2H4��ȫȼ�շų�����������д���Ȼ�ѧ����ʽ��
(1)��Ԫ�ص�ԭ�Ӻ�����7�����ӣ������������ԭ����K����2�����ӣ�L����5��������
(2) ��������Ԫ����-1�ۣ����µķ���ʽN2H4��֪����Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����NaClO���н�ǿ�������ԣ���Ӧ��ͨ����Ԫ����+1�۱仯��-1�ۣ��õ����ӣ�NH3ת��ΪN2H4�Ĺ����е�Ԫ�صĻ��ϼ���-3�۱仯��-2����ʧȥ���������Ը÷�ӦΪ������ԭ��Ӧ��1��NaClOת��Ϊ1��NaCl�õ�2�����ӣ�2��NH3����1���·���ʧȥ2�����ӣ����ݵ��ӵ�ʧ�غ��ԭ���غ���ƽ���仯ѧ��Ӧ����ʽΪ��2NH3+NaClO = N2H4 + NaCl + H2O�����µ�Ħ������=32g/mol��16gN2H4�����ʵ���=![]() =0.5mol��1molN2H4��ȫȼ�շų�������=267.1kJ��
=0.5mol��1molN2H4��ȫȼ�շų�������=267.1kJ��![]() =534.2kJ�����Ȼ�ѧ����ʽΪ��N2H4(l)+O2(g)=N2(g)+2H2O(g)����H=-534.2kJmol-1��
=534.2kJ�����Ȼ�ѧ����ʽΪ��N2H4(l)+O2(g)=N2(g)+2H2O(g)����H=-534.2kJmol-1��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�����й������ʷ������ȷ����� ( )
�� | �� | �� | ���������� | ���������� | |
A | Na2CO3 | H2SO4 | (NH4)2CO3 | MgO | CO2 |
B | NaOH | HCl | NaCl | Na2O | H2O |
C | Ba(OH)2 | H2CO3 | CaCl2 | CO2 | SO2 |
D | KOH | HNO3 | CaCO3 | CaO | SO3 |
A. A B. B C. C D. D