��Ŀ����
(8��)�⻯����ʵ�����г��õķ����Լ�����ҵ������м��ԭ���Ʊ�NaI����������ͼ��
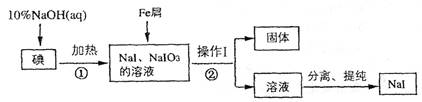
��ش��������⣺
(1) �жϷ�Ӧ���е��Ƿ�Ӧ��ȫ�ķ�����_____________________
(2) ����I��������_____________________��
(3) ��Ӧ�ٵ����ӷ���ʽΪ_____________________
(4) ��Ӧ����NaIO3��Fe���ʻ�ԭΪNaI��ͬʱ����Fe(OH)3,�÷�Ӧ�Ļ�ѧ����ʽ��______��
�ڸ÷�Ӧ������99 g NaIO3����ԭ����ת�Ƶ��ӵ����ʵ���Ϊ_______mol

��ش��������⣺
(1) �жϷ�Ӧ���е��Ƿ�Ӧ��ȫ�ķ�����_____________________
(2) ����I��������_____________________��
(3) ��Ӧ�ٵ����ӷ���ʽΪ_____________________
(4) ��Ӧ����NaIO3��Fe���ʻ�ԭΪNaI��ͬʱ����Fe(OH)3,�÷�Ӧ�Ļ�ѧ����ʽ��______��
�ڸ÷�Ӧ������99 g NaIO3����ԭ����ת�Ƶ��ӵ����ʵ���Ϊ_______mol
��8�֣���1��ȡ������Ӧ�����Һ���Թ��У����뼸�ε�����Һ������Һδ��������֤�����ѷ�Ӧ��ȫ����֮����δ��Ӧ��ȫ����1�֣�����ȡ������Ӧ�����Һ���Թ��У����뼸��CCl4�������ã����²�Һ�����ɫ��֤�����ѷ�Ӧ��ȫ�����²�Һ����Ϻ�ɫ��֤����δ��Ӧ��ȫ����
��2�����ˣ�1�֣� ��3��3I2 + 6OH��=5I��+ IO3��+ 3H2O ��2�֣�
��4��2Fe+ NaIO3+ 3H2O=2Fe(OH)3��+NaI ��2�֣� 3��2�֣�
��2�����ˣ�1�֣� ��3��3I2 + 6OH��=5I��+ IO3��+ 3H2O ��2�֣�
��4��2Fe+ NaIO3+ 3H2O=2Fe(OH)3��+NaI ��2�֣� 3��2�֣�
���⿼�鹤ҵ�����й�֪ʶ����1�����ʵ������۱�������˿��õ�����Һ���顣Ҳ�ɲ�����ȡ�ķ��������ⵥ����ȡ����������ȡ���е���ɫ����ȡ������Ӧ�����Һ���Թ��У����뼸�ε�����Һ������Һδ��������֤�����ѷ�Ӧ��ȫ����֮����δ��Ӧ��ȫ����ȡ������Ӧ�����Һ���Թ��У����뼸��CCl4�������ã����²�Һ�����ɫ��֤�����ѷ�Ӧ��ȫ�����²�Һ����Ϻ�ɫ��֤����δ��Ӧ��ȫ����(2)������ͼ��������I�����ƺ���õ����ǹ������Һ���ʲ���I�������ǹ��ˡ�(3)�ӷ�Ӧǰ����Կ�������Ӧ��ΪNaOH��I2������ΪNaI��NaIO3���ʷ�Ӧ�����ӷ���ʽΪ3I2 + 6OH��=5I��+ IO3��+ 3H2O����4��NaIO3��Fe���ʻ�ԭΪNaI��ͬʱ����Fe(OH)3,�÷�Ӧ�Ļ�ѧ����ʽ��2Fe+ NaIO3+ 3H2O=2Fe(OH)3��+NaI���ӷ���ʽǰ�ϼ۵������������ڸ÷�Ӧ������99 g NaIO3(��0.5mol)����ԭΪNaI(�õ�6mol����)����ת�Ƶ��ӵ����ʵ���Ϊ3mol

��ϰ��ϵ�д�
 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
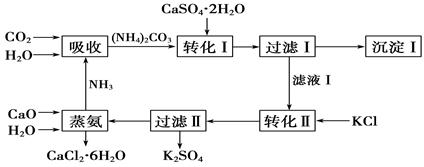
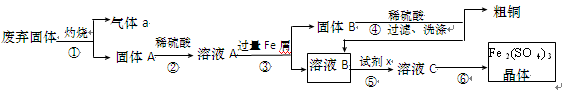
�����Ŀ
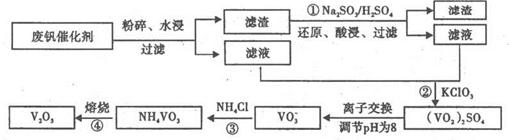

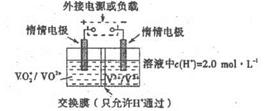
 VO2����V3����H2O
VO2����V3����H2O