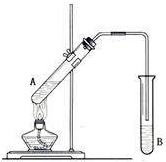
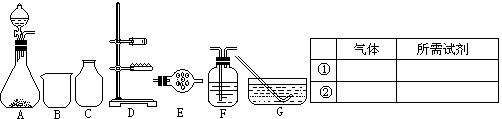
��Ŀ����
���Ѿ���ȡ�Ȼ��ơ��塢þ�Ȼ�ѧ���ʵĸ���±ˮ�У���������Ĺ��������������ʵ⣺
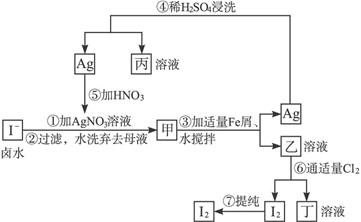
������������⣺
(1)�ҡ��������ʵĻ�ѧʽ����____________����____________��
(2)�ڢܲ���������ϡH2SO4��ϴ��Ŀ����____________(��д��ĸ���)��
a.��ȥδ��Ӧ��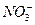 b.��ȥδ��Ӧ��I-
b.��ȥδ��Ӧ��I-
c.��ȥδ��Ӧ��Fe d.��ȥ��������
(3)�ڢ߲������ɹ��ᴿI2��һ�ַ�����____________(��Ҫ��д���岽��)��
(4)ʵ���Ҽ���I2�ķ�����________________________��
(5)�����ʼ����ױ�ڣ���ԭ����______________________________(�û�ѧ����ʽ��ʾ)��

������������⣺
(1)�ҡ��������ʵĻ�ѧʽ����____________����____________��
(2)�ڢܲ���������ϡH2SO4��ϴ��Ŀ����____________(��д��ĸ���)��
a.��ȥδ��Ӧ��
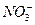 b.��ȥδ��Ӧ��I-
b.��ȥδ��Ӧ��I-c.��ȥδ��Ӧ��Fe d.��ȥ��������
(3)�ڢ߲������ɹ��ᴿI2��һ�ַ�����____________(��Ҫ��д���岽��)��
(4)ʵ���Ҽ���I2�ķ�����________________________��
(5)�����ʼ����ױ�ڣ���ԭ����______________________________(�û�ѧ����ʽ��ʾ)��
(1)FeI2 FeCl3 (2)c (3)��������ȡ (4)��I2�ӵ�������Һ�У���Һ����ɫ (5)2AgI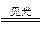 2Ag+I2
2Ag+I2
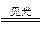 2Ag+I2
2Ag+I2����±ˮ�е�I-��Ag+��Ӧ����AgI(��)��AgI��������ˮ�о����Ͻ��裬�Ỻ��������Ӧ������Ag��FeI2(��������)���ڵڢܲ�����ϡH2SO4���ܽ��������۶��ᴿ�����ٰ����������ᣬ��ȡAgNO3��������I-��Ӧ��������Һ��ͨ������Cl2��������FeCl3��Һ(��)��I2���ʣ�����������ȡ�İ취�ᴿ�⡣
���������⿼����±�ؼ��仯��������ԡ�X-(±����)�ļ����֪ʶ��������ѧ���ۺ�����֪ʶ��������
������ƽ���ƶ��Ĺ۵����AgI��������ˮ�еķ�Ӧ��AgI(s) Ag++I-������Fe��2Ag++Fe====2Ag+Fe2+��ʹAgI���ܽ�ƽ�������ƶ���AgI���ܽ�����FeI2��Ag��
Ag++I-������Fe��2Ag++Fe====2Ag+Fe2+��ʹAgI���ܽ�ƽ�������ƶ���AgI���ܽ�����FeI2��Ag��
���������⿼����±�ؼ��仯��������ԡ�X-(±����)�ļ����֪ʶ��������ѧ���ۺ�����֪ʶ��������
������ƽ���ƶ��Ĺ۵����AgI��������ˮ�еķ�Ӧ��AgI(s)
 Ag++I-������Fe��2Ag++Fe====2Ag+Fe2+��ʹAgI���ܽ�ƽ�������ƶ���AgI���ܽ�����FeI2��Ag��
Ag++I-������Fe��2Ag++Fe====2Ag+Fe2+��ʹAgI���ܽ�ƽ�������ƶ���AgI���ܽ�����FeI2��Ag��
��ϰ��ϵ�д�
�����Ŀ
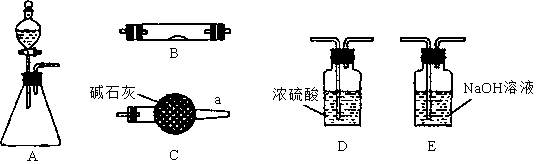
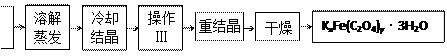 K2C2O4
K2C2O4