��Ŀ����
�����ӵ�����ԼΪ6.02��1023 mol��1��������������ȷ����( )
| A��1.01��105 Pa��25 ��ʱ��2.24 L Cl2�к��е�ԭ����Ϊ0.2��6.02��1023 |
| B��0.1 L 3 mol��L��1 NH4NO3��Һ�к��е�Nԭ����ĿΪ0.3��6.02��1023 |
| C��5.6 g����������CuSO4��Һ��Ӧ���ɵ�ͭԭ����Ϊ1��6.02��1023 |
| D��46 g NO2��N2O4�Ļ�����к��е�ԭ����Ϊ3��6.02��1023 |
D
�������������A1.01��105 Pa��25 ��ʱ��2.24 L Cl2�����ʵ���С��0.1mol,�����е�ԭ��������0.2��6.02��1023������Bÿ�� NH4NO3����2��Nԭ�ӣ���0.1 L 3 mol��L��1 NH4NO3��Һ�к��е�Nԭ����ĿΪ2��0.3��6.02��1023������C��5.6 g�������ʵ���Ϊ0.1mol.ÿ����ԭ��ʧȥ2�����ӡ���������������CuSO4��Һ��Ӧ���ɵ�ͭԭ����Ϊ0.2��6.02��1023.����D��NO2��Է�������Ϊ46��46 g NO2�����ʵ���Ϊ1mol����ԭ��3mol. N2O4��Է�������Ϊ92.46g N2O4���ʵ���0.5mol.����ԭ�ӵ����ʵ���Ϊ0.5��6=3mol.����46 g NO2��N2O4�Ļ�����к��е�ԭ����Ϊ3��6.02��1023����ȷ��D
���㣺���鰢���ӵ��������йؼ����֪ʶ��

��NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ� ��
| A��64g SO2������ԭ����ΪNA |
| B��0.5mol/L MgCl2��Һ������Cl-������ΪNA |
| C����״���£�22.4L H2O�ķ�����ΪNA |
| D�����³�ѹ�£�14g N2���з�����Ϊ0.5NA |
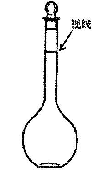
������ҺCl�������ʵ���Ũ����50 mL 1 mol��L��1�Ȼ�����Һ��Cl�������ʵ���Ũ����ȵ���
| A��150 mL 1 mol��L��1�Ȼ�����Һ | B��75 mL 1.5 mol��L��1�Ȼ�����Һ |
| C��150 mL 1 mol��L��1�Ȼ�����Һ | D��50 mL 3 mol��L��1�Ȼ�þ��Һ |
ͬ��ͬѹ�£��������Բ��Ƶ�������A��B���ֱ����X�����Y���壬�ҳ�����������������ͬ������ͬ�����£�A�������CO�о�ֹ������B�������O2���ϸ��������������ʾ��ȷ���ǣ� ��
| A��X�������Է���������Y�������Է��������� |
| B��X������N2��Y������CH4 |
| C��X������ܶ�С��Y������ܶ� |
| D��������A����������B���������� |
��MgSO4��Al2(SO4)3�Ļ����Һ�У����Mg2+���ʵ���Ũ��Ϊ0.1mol/L��
���ʵ���Ũ��Ϊ0.4mol/L��������Һ��Al3+���ʵ�����Ũ��Ϊ�� ��
| A��0.2mol/L | B��0.15mol/L | C��0.1mol/L | D��0.05mol/L |
��2��3g�Ʒ�������������Ϊm g��ˮ�У�����0��1molþ��������������Ϊm g��ϡ�����У���Ӧ�����������Һ�������ֱ�Ϊa g��b g ����a g��b g�����Ĺ�ϵӦ��
| A��a��b | B��a��b | C��a��b | D����ȷ�� |
�����������ͬ��ijֲ���Ӫ��Һ�����䷽���±���ʾ��
| | KCl | K2SO4 | ZnSO4 | ZnCl2 |
| 1 | 0��3mol | 0��2mol | 0��1mol | �� |
| 2 | 0��1mol | 0��3mol | �� | 0��1mol |
��������Ӫ��Һ�ijɷ֣�����˵���У���ȷ����( )
A��ֻ��n(K��)��ͬ B��ֻ��n(Cl��)��ͬ
C�������ӵ����ʵ�������ͬ D�������ӵ����ʵ�����ȫ��ͬ
���л�ѧ�����������ȷ����
A�����ӽṹʾ��ͼ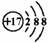 �����Ա�ʾ35Cl����Ҳ���Ա�ʾ37Cl�� �����Ա�ʾ35Cl����Ҳ���Ա�ʾ37Cl�� |
B������ʽ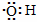 �����Ա�ʾ�ǻ���Ҳ���Ա�ʾ���������� �����Ա�ʾ�ǻ���Ҳ���Ա�ʾ���������� |
C������ģ�� �����Ա�ʾ������ӣ�Ҳ���Ա�ʾ���Ȼ�̼���� �����Ա�ʾ������ӣ�Ҳ���Ա�ʾ���Ȼ�̼���� |
D���۱�ϩ�Ľṹ��ʽ��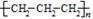 |