��Ŀ����
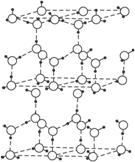
 ˮ���Ӽ����һ�ֽС�����������ã����ڷ����߶�˹���뻯ѧ��֮�䣩�˴˽�϶��γ�(H2O)n���ڱ���ÿ��ˮ���ӱ�4��ˮ���Ӱ�Χ�γɱ��ε��������壬ͨ�����������ӳ��Ӵ�ķ��Ӿ���һ�Σ���ṹʾ��ͼ��ͼ��ʾ
ˮ���Ӽ����һ�ֽС�����������ã����ڷ����߶�˹���뻯ѧ��֮�䣩�˴˽�϶��γ�(H2O)n���ڱ���ÿ��ˮ���ӱ�4��ˮ���Ӱ�Χ�γɱ��ε��������壬ͨ�����������ӳ��Ӵ�ķ��Ӿ���һ�Σ���ṹʾ��ͼ��ͼ��ʾ
��1mol������ mol�������
��ˮ���ӿɵ����������ֺ�����ͬ����������������뷽��ʽΪ�� ��
���ڱ��Ľṹ�У�ÿ��ˮ���������ڵ�4��ˮ��������������ӡ��ڱ������г�����⣬�����ڷ����߶�˹����11kJ•mol�D1������֪������������51 kJ•mol�D1���������������������� kJ•mol�D1
����x��y��z�ֱ��ʾH2O��H2S��H2Se�ķе㣨�棩����x��y��z�Ĵ�С��ϵ�� �����ж�������__________________________________________��
(1) 2 (2) H2O + H2O ![]() H3O+ + OH- (3) 20 (4) x �� z �� y ˮ�к�������ʷе���ߣ��������������Է����������Ӽ���������������е�ߡ�
H3O+ + OH- (3) 20 (4) x �� z �� y ˮ�к�������ʷе���ߣ��������������Է����������Ӽ���������������е�ߡ�

��ϰ��ϵ�д�
�����Ŀ