��Ŀ����
(12��) ����ʯ����Ҫ�ɷ���K2SO4��Al2(SO4)3��2Al 2O3��6H2O�������������Fe2O3���ʡ���������ʯ�Ʊ������������������£�
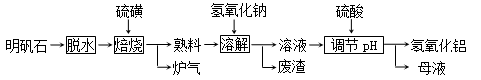
�ش��������⣺
��1�������ա������з����ķ�ӦΪ2Al2(SO4)3��3S 2Al 2O3��9SO2������������Ϊ ��
2Al 2O3��9SO2������������Ϊ ��
��2�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ ��
��3��������pH������ˡ�ϴ��Al(OH)3������֤����ϴ�Ӹɾ���ʵ�������������
��
��4������pHʱʹ�õ�������Ũ�ȣ���λ�����Һ�������ʵ�������Ϊ882 g/L ��H2SO4 ������1L����Һ��������Ͳ��ȡ��������Ϊ98�������ᣨ�ܶ���1.8g/cm3��
mL
��5��������������ĸҺ���пɻ��յ����ʷֱ��� ��

�ش��������⣺
��1�������ա������з����ķ�ӦΪ2Al2(SO4)3��3S
 2Al 2O3��9SO2������������Ϊ ��
2Al 2O3��9SO2������������Ϊ ����2�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ ��
��3��������pH������ˡ�ϴ��Al(OH)3������֤����ϴ�Ӹɾ���ʵ�������������
��
��4������pHʱʹ�õ�������Ũ�ȣ���λ�����Һ�������ʵ�������Ϊ882 g/L ��H2SO4 ������1L����Һ��������Ͳ��ȡ��������Ϊ98�������ᣨ�ܶ���1.8g/cm3��
mL
��5��������������ĸҺ���пɻ��յ����ʷֱ��� ��
��12�֣���1��Al2(SO4)3��2�֣� ��2��Al2O3��2OH����2AlO2����H2O ��2�֣�
��3��ȡ���һ��ϴ�ӵ�����Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ������������ϴ�Ӹɾ�����2�֣� ��4��500.0 mL��3�֣�
��5��Fe2O3��K2SO4��Na2SO4��3�֣�
��3��ȡ���һ��ϴ�ӵ�����Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ������������ϴ�Ӹɾ�����2�֣� ��4��500.0 mL��3�֣�
��5��Fe2O3��K2SO4��Na2SO4��3�֣�
����Ϊ���������⣬�漰�������̡�ʵ���������Ӧ���۵ȡ���1�����չ����з�������һ�����з�ӦS��+6��0��+4�ۣ��õ���������K2SO4��Al2O3����NaOH�ܽ⣬��Al2O3ת��ΪNaAlO2��Ȼ�����pH��AlO+H++H2O=Al(OH)3��882g/L�����ᣬ���ʵ���Ũ��Ϊ9mol/L��98%������Ϊ18mol/L���Ӷ�������Ҫ���Ϊ500ml��

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ
