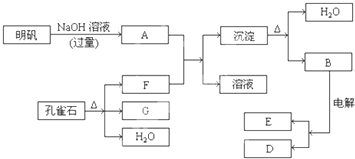
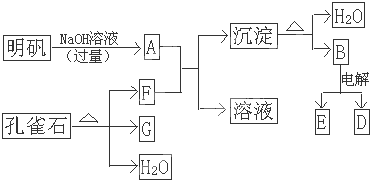
��Ŀ����
 ��һ�������¿�ʵ����ͼ��ʾ����֮��ı仯��
��һ�������¿�ʵ����ͼ��ʾ����֮��ı仯������д���¿հף�
��1����ȸʯ����Ҫ�ɷ���CuCO3?Cu��OH��2����ʽ̼��ͭ���������ֽ⣮��ͼ�е�F��
CO2���������̼��
CO2���������̼��
����2��д��������Һ�����NaOH��Һ��Ӧ�����ӷ���ʽ��
Al3++4OH-=AlO2-+2H2O
Al3++4OH-=AlO2-+2H2O
����3��ͼ������G��D��Ϊ���壬��ͺ��ڸ����¿ɷ�����Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ��
3CuO+2Al
3Cu+Al2O3
| ||
3CuO+2Al
3Cu+Al2O3
��
| ||
��4��ÿ����1ĦD��ͬʱ����
0.75
0.75
ĦE����������1����ȸʯ����Ҫ�ɷ���CuCO3?Cu��OH��2����ʽ̼��ͭ���������ֽ�IJ���Ϊ����ͭ��ˮ��������̼��
��2����������Ҫ�ɷ���KAl��SO4��2?12H2O���������ܺ���NaOH��Һ��Ӧ��
��3���������ڸ��������û�������ͭ�е�ͭ��
��4����ҵ�ϲ��õ�����ڵ��������ķ�������ȡ������Ӧ����ʽΪ��2Al2O3
4Al+3O2����
��2����������Ҫ�ɷ���KAl��SO4��2?12H2O���������ܺ���NaOH��Һ��Ӧ��
��3���������ڸ��������û�������ͭ�е�ͭ��
��4����ҵ�ϲ��õ�����ڵ��������ķ�������ȡ������Ӧ����ʽΪ��2Al2O3
| ||
����⣺��1����ȸʯ����Ҫ�ɷ���CuCO3?Cu��OH��2����ʽ̼��ͭ���������ֽ�IJ���Ϊ����ͭ��ˮ��������̼����������Ҫ�ɷ���KAl��SO4��2?12H2O�����е��������ܺ���NaOH��Һ��Ӧ����ƫ������ˮ��Һ������AΪ��NaAlO2����ʽ̼��ͭ�����ֽ�IJ����У�ֻ�ж�����̼���Ժ�A��Ӧ������FΪ��CO2���ʴ�Ϊ��CO2���������̼����
��2��������Һ�����NaOH��Һ��Ӧ��ʵ���ǣ������Ӻ�������֮��ķ�Ӧ�����ӷ���ʽΪ��Al3++4OH-=AlO2-+2H2O���ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
��3��GΪ����ͭ��BΪ�����������ȷֽ�IJ���֮һ��Ϊ��������������Ϊ������������ͭ�����µķ�ӦΪ��3CuO+2Al
3Cu+Al2O3���ʴ�Ϊ��3CuO+2Al
3Cu+Al2O3��
��4�����������ķ�ӦΪ��2Al2O3
4Al+3O2��������������ԭ��Ӧ��ͼ������G��D��Ϊ���壬����DΪ����ÿ����1Ħ����ͬʱ����0.75mol���������ʴ�Ϊ��0.75mol��
��2��������Һ�����NaOH��Һ��Ӧ��ʵ���ǣ������Ӻ�������֮��ķ�Ӧ�����ӷ���ʽΪ��Al3++4OH-=AlO2-+2H2O���ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
��3��GΪ����ͭ��BΪ�����������ȷֽ�IJ���֮һ��Ϊ��������������Ϊ������������ͭ�����µķ�ӦΪ��3CuO+2Al
| ||
| ||
��4�����������ķ�ӦΪ��2Al2O3
| ||
������������һ����ͼ�Ƶ��⣬������ѧ���������ʵ����ʣ�Ҫ��ѧ����ǽ̲�֪ʶ��ѧ�����ã�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ