��Ŀ����
����Ŀ��ij���Ը���������![]() ��Ҫ��Al2O3��Fe2O3��SiO2��������FeS2��
��Ҫ��Al2O3��Fe2O3��SiO2��������FeS2��![]() ��ȡ�������ʹ�����������ֱ�Ӽ��ܷ������γ��������Ƴ���[NamAlmSinO16(OH)5]���������ʧ��һ�ָĽ�����������£�
��ȡ�������ʹ�����������ֱ�Ӽ��ܷ������γ��������Ƴ���[NamAlmSinO16(OH)5]���������ʧ��һ�ָĽ�����������£�
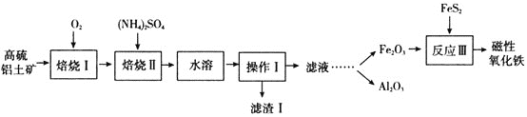
������Ԫ�����ڱ��е�λ����________________��NamAlmSinO16(OH)5�е�m��n֮������ʲô���Ĵ���ʽ________��д����������Ҫ�ɷֵ�һ����;��________________________����Ӧ������FeS2��Ŀ������Ϊ________________________![]() ������������������ԭ����
������������������ԭ����![]() ��
��
�Ʊ����������л������������ɫ�̳���SO2���壬д��������һ����Ļ�ѧ����ʽ��________________________________________________��
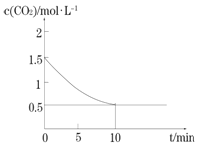
�DZ����������������������Ƶÿ����Ե�NH4Al(SO4)2��NH4Fe(SO4)2����ȡ�����¶ȡ�ʱ��仯������ͼ��ʾ����ѵı���ʱ�����¶���________________������NH4R(SO4)2��ʾNH4Al(SO4)2��NH4Fe(SO4)2����صĻ�ѧ��Ӧ����ʽΪ________________________��
�������й���������[NamAlmSinO16(OH)5]������Ԫ�����ʵ�˵����ȷ����________��
A.��Ϊԭ�Ӱ뾶��Na > S���������Ӱ뾶��Na+ > S2��
B.��Ϊ�ǽ����ԣ�S > Si�����Լ���̬�⻯���ȶ��ԣ�SiH4 < H2S
C.��Ϊ�ǽ����ԣ�O > S�����Էе㣻 H2S > H2O
D.��Ϊ�����ԣ�Na > Al�����Լ��ԣ�NaOH > Al(OH)3
��Ϊ�˲ⶨWg��������������Ԫ�صĺ���������������ȡ��Al2O3�ܽ�������ϡ���ᣬ���250mL��Һ��ȡ��25mL������c mol��L��1 EDTA����ҺamL��������ҺpHԼΪ4.2����У���ȴ����b mol��L��1 CuSO4����Һ�ζ�������EDTA���յ㣬����CuSO4����ҺV mL(��֪Al3+��Cu2+��EDTA��Ӧ�Ļ�ѧ�����Ⱦ�Ϊ1:1)����Wg��������������Ԫ�ص���������Ϊ________________________![]() �ú�V��W��a��b��c�Ĵ���ʽ��ʾ
�ú�V��W��a��b��c�Ĵ���ʽ��ʾ![]() ��
��
���𰸡���������VIII�� 4m + 4n =37 �Ʋ����� ��ԭ�� 4 FeS2 + 11O2![]() 8SO2+ 2 Fe2O3 60min��450������ R2O3 +4 (NH4)2SO4
8SO2+ 2 Fe2O3 60min��450������ R2O3 +4 (NH4)2SO4 ![]() 2NH4R(SO4)2 + 6NH3��+ 3H2O BD
2NH4R(SO4)2 + 6NH3��+ 3H2O BD ![]()
��������
������Ԫ�����ڱ��е�λ���ǵ�������VIII�壻����NamAlmSinO16(OH)5���ϼ۴�����Ϊ0���ó�m��n֮���ϵ����������Ҫ�ɷ��Ƕ������裬�����Ʋ����ȣ���Ӧ��������������FeS2��Ӧ�����������������������������ϼ۲��ֽ��ͣ�FeS2���ϼ����ߡ�
�������������л������������ɫ�̳���SO2���壬����������ԭ��Ӧд����ѧ����ʽ��
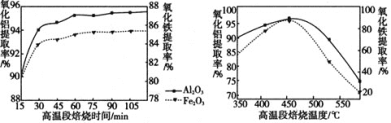
������ͼ��ó�������ʱ����60min��ȡ�ʻ����ﵽ�Ƚϴ��ֵ���ٳ������ȣ���ȡ�����Ӻ��٣��������˳ɱ�����˱���ʱ��60min���¶���450�����ң���ȡ������ݷ�Ӧԭ��д����صĻ�ѧ��Ӧ����ʽ��
��A����Ϊԭ�Ӱ뾶��Na > S����ྶ��Ƚϰ뾶��
B����Ϊ�ǽ����ԣ�S > Si�������ң�����̬�⻯���ȶ���Խ��Խǿ��
C����Ϊ�ǽ����ԣ�O > S��ˮ�д��ڷ��Ӽ������
D����Ϊ�����ԣ�Na > Al�������Ҽ���������
������Al3+��Cu2+��EDTA��Ӧ�Ļ�ѧ�����Ⱦ�Ϊ1:1�������n(Al3+)���ٸ��������������м��㡣
������Ԫ�����ڱ��е�λ���ǵ�������VIII�壻����NamAlmSinO16(OH)5���ϼ۴�����Ϊ0���ó�(+1)��m+(+3)��m+(+4)��n+(-2)��16+(-1)��5 = 0��m��n֮������4m + 4n =37����������Ҫ�ɷ��Ƕ������裬�����Ʋ����ȣ���Ӧ��������������FeS2��Ӧ�����������������������������ϼ۲��ֽ��ͣ�FeS2���ϼ����ߣ�Ŀ������Ϊ��ԭ����
�ʴ�Ϊ����������VIII�壻4m + 4n =37���Ʋ����ȣ���ԭ����
�������������л������������ɫ�̳���SO2���壬�仯ѧ����ʽ��4 FeS2 + 11O2![]() 8SO2+ 2 Fe2O3��
8SO2+ 2 Fe2O3��
�ʴ�Ϊ��4FeS2 + 11O2![]() 8SO2+ 2 Fe2O3��
8SO2+ 2 Fe2O3��
������ͼ��ó�������ʱ����60min��ȡ�ʻ����ﵽ�Ƚϴ��ֵ���ٳ������ȣ���ȡ�����Ӻ��٣��������˳ɱ�����˱���ʱ��60min���¶���450�����ң���ȡ����������ѵı���ʱ�����¶���60min��450�����ң���NH4R(SO4)2��ʾ����صĻ�ѧ��Ӧ����ʽΪR2O3 +4 (NH4)2SO4![]() 2NH4R(SO4)2 + 6NH3 + 3H2O��
2NH4R(SO4)2 + 6NH3 + 3H2O��
�ʴ�Ϊ��60min��450�����ң�R2O3 +4 (NH4)2SO4 ![]() 2NH4R(SO4)2 + 6NH3 + 3H2O��
2NH4R(SO4)2 + 6NH3 + 3H2O��
��A����Ϊԭ�Ӱ뾶��Na > S����ྶ��������Ӱ뾶�� S2��> Na+����A����
B����Ϊ�ǽ����ԣ�S > Si�������ң�����̬�⻯���ȶ���Խ��Խǿ����SiH4 < H2S����B��ȷ��
C����Ϊ�ǽ����ԣ�O > S��ˮ�д��ڷ��Ӽ��������˷е㣻 H2O >H2S����C����
D����Ϊ�����ԣ�Na > Al�������Ҽ�����������˼��ԣ�NaOH > Al(OH)3����D��ȷ��
�ʴ�ΪBD��
������Al3+��Cu2+��EDTA��Ӧ�Ļ�ѧ�����Ⱦ�Ϊ1:1���ó�n(Al3+)= c mol��L��1 ��a��10-3L��b mol��L��1 ��V��10-3L = (ac ��bV) ��0-3mol����Wg��������������Ԫ�ص���������Ϊ![]() ��
��
�ʴ�Ϊ��![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�