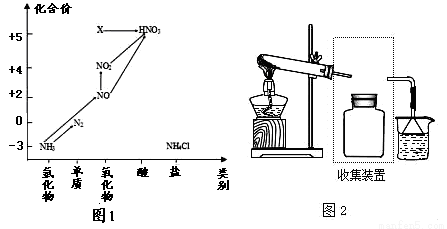
��Ŀ����
������ij��ѧ��ѧʵ��С����ʵ���Ҽ���ij��ɫ��Һ����֪��Һ�е����ʽ���NH4+��K+��Ag+��Ca2+��Al3+��AlO2-��MnO4-��CO32-��SO42-�е���������ɡ�ȡ����Һ��������ʵ�飺
��1��ȡ������Һ������������ᣬ���������ɣ����õ���ɫ��Һ
��2���ڣ�1��������Һ�м������NH4HCO3��Һ�����������ɣ�ͬʱ������ɫ�����ף�
��3���ڣ�2��������Һ�м������Ba��OH��2��Һ������Ҳ���������ɣ�ͬʱ������ɫ������
������������ԭ��Һ��һ�����ڵ���
A. K+��CO32-��AlO2-
B. SO42-��AlO2-��K+��CO32-
C. CO32-��K+��Al3+��NH4+
D. MnO4-��K+��CO32-��NH4+
����/H2O2��Һ����������������������
(1)��֪T��ʱ��2SO2(g)+O2(g)  2SO3(g) ��H1
2SO3(g) ��H1
2H2O2(I)=2H2O(I)+O2(g) ��H2
SO3(g)+H2O(I))=H2SO4(I) ��H3
��SO2(g)+H2O2(I))=H2SO4(I) ��H4=__________ (�ú���H1����H2����H3�Ĵ���ʽ��ʾ)
(2)����[CO(NH2)2]��Һ��NOx��SO2��һ�����ѳ��ʡ���SO2��NOx (N��Լռ90%)ͨ���������������Ϊ7%������Ũ��Ϊ5%�ķ�Ӧ���н��з�Ӧ��
��������SO2����ת��Ϊһ�����Σ��仯ѧʽΪ______________��NO��NO2�����ʵ���֮��1:1��CO(NH2)2��Ӧ����������Ļ�ѧ����ʽΪ_______________��
�ڰ��������(H2NCOONH4)�����ص�ˮ������һ�����İ�����������ں����ܱ������У�������Ӧ��NH2COONH4(s) 2NH3(g)��CO2(g) ��H��ʵ���ò�ͬ�¶���ƽ��ʱ�������Ũ�����±���
2NH3(g)��CO2(g) ��H��ʵ���ò�ͬ�¶���ƽ��ʱ�������Ũ�����±���
�¶�/K | 338 | 343 | 348 | 353 |
ƽ��ʱ�������Ũ��/mol • L-1 | 0.36 | 0.48 | 0.60 | 0.72 |
�÷�Ӧ�ġ�H__________(�>����<��)0��348Kʱ���÷�Ӧ��ƽ�ⳣ��K__________________��
(3)�����������䣬��������Һ������H2O2��Һ����ò�ͬ pH�µ���������ѳ�����ʱ��Ĺ�ϵ��ͼ��ʾ��
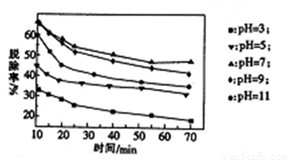
��NO��H2O2������Ӧ����������ʱ���������뻹ԭ�������ʵ���֮��Ϊ____________��
(2)����ʱ�����pHΪ_________���ڼ��Խ�ǿʱ��NOx�ѳ��ʽ��ͣ���ԭ����___________(��дһ��)��





 Na++ OH-
Na++ OH-

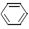 + H2(g) ��
+ H2(g) �� 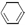 (l) ��H��0 �٣�
(l) ��H��0 �٣� 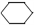 (l) ��H��0 �ڡ�
(l) ��H��0 �ڡ�