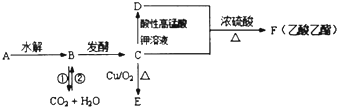
��Ŀ����
2����ˮAlCl3�������л��ϳɵĴ�����ʳƷ���ɼ��ȣ���ҵ�Ʊ���ˮAlCl3���������£�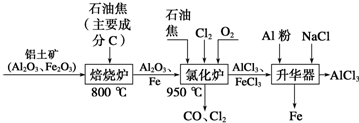
��1���Ȼ�¯��ͨ��O2��Ŀ������C��Ӧ���ṩ��Ӧ�����������
��2���Ȼ�¯��Al2O3��Cl2��C��Ӧ�Ļ�ѧ����ʽ��Al2O3+3Cl2+3C$\frac{\underline{\;����\;}}{\;}$2AlCl3+3CO��
��3���Ȼ�¯�е��������壬ͨ��������������Һ�����գ���д����Na2SO3��Һ����β��ʱ������Ӧ�����ӷ���ʽSO32-+C12+H2O�TSO42-+2C1-+2H+����
��4�����������м������۵�Ŀ���dz�ȥFeCl3��
��5�����Ȼ������壨AlCl3•6H2O����HCl���м���Ҳ�ܽ�����ˮ�Ȼ������Ʊ�������ʵ���������Ʋ��������յõ��������Ǽ�ʽ�Ȼ���[��ѧʽΪAl2��OH��nCl��6-n��]����������ԭ�Ȼ������壨AlCl3•6H2O����40%���������n��ֵΪ4��
��6�����������У�������Ϊ�����������ε��Һ����Ԫ����Ҫ��AlCl${\;}_{4}^{-}$��ʽ���ڣ��������ĵ缫��ӦʽΪAl-3e-+4Cl-�TAlCl4-��
���� ��1���Ȼ�¯��ͨ��O2����̼��Ӧ���ȣ�ά��950����¶ȣ�
��2�����ݹ������̿�֪�������к���AlCl3�ȣ�����Al2O3��C12��C��Ӧ������A1C13����������β����֪��������CO��
��3��Cl2��ǿ�����ԣ���SO32-����ΪSO42-����������ԭΪ2C1-��
��4������������Ҫ����AlCl3��FeCl3��FeCl3�۵㡢�е�ϵͣ������Ʊ���ˮAlCl3��Ӧ��ȥFeCl3��
��5�����ݼ�ʽ�Ȼ������Ȼ��������40%����ʽ���㣻
��6�����������У��������������ŵ�����AlCl4-��
��� �⣺��1���Ȼ�¯��ͨ��O2����̼��Ӧ���ȣ�ά��950����¶ȣ��ʴ�Ϊ����C��Ӧ���ṩ��Ӧ�����������
��2�����ݹ������̿�֪�Ȼ�¯�IJ������ȴ���������Ʊ���ˮAlCl3��˵���Ȼ�¯�IJ����к���A1C13��������β������CO������Al2O3��C12��C��Ӧ������A1C13��CO����Ӧ����ʽΪA12O3+3C12+3C$\frac{\underline{\;����\;}}{\;}$2A1C13+3CO���ʴ�Ϊ��A12O3+3C12+3C$\frac{\underline{\;����\;}}{\;}$2A1C13+3CO��
��3��Cl2��ǿ�����ԣ���SO32-����ΪSO42-����������ԭΪ2C1-����Ӧ���ӷ���ʽΪSO32-+C12+H2O�TSO42-+2C1-+2H+���ʴ�Ϊ��SO32-+C12+H2O�TSO42-+2C1-+2H+��
��4������������Ҫ����AlCl3��FeCl3��FeCl3�۵㡢�е�ϵͣ������Ʊ���ˮAlCl3��Ӧ��FeCl3ȥ�����Լ�������AlĿ���dz�ȥFeCl3���ʴ�Ϊ����ȥFeCl3��
��5�����ݼ�ʽ�Ȼ������Ȼ��������40%����ʽ241.5��40%=54+17n+213-35.5n��n=4��
�ʴ�Ϊ��96%��
��6�����������У��������������ŵ�����AlCl4-��Al-3e-+4Cl-�TAlCl4-���ʴ�Ϊ��Al-3e-+4Cl-�TAlCl4-��
���� ���⿼�黯ѧ����ʽ���ӷ���ʽ����д�����ʵķ�����ᴿ�����ȵȣ���Ŀ�Ѷ����У�ע�⽫AlCl3•6H2O��ˮ�Ʊ���ˮ�Ȼ�������HCl�����м����ѽᾧˮ��

| A�� | 2��5-����-4-�һ����� | B�� | 2-��-1-��Ȳ | ||
| C�� |  2-��-2-�ȱ��� 2-��-2-�ȱ��� | D�� |  2-��-1-���� 2-��-1-���� |
| A�� | ��С���������ʹѹǿ���� | B�� | ������䣬����N2 | ||
| C�� | ���ݣ�����He | D�� | ���ͷ�Ӧ�¶� |
| A�� | C2H2��BeCl2 | B�� | SiCl4��NH4+ | C�� | H2S��P4 | D�� | CH4��BF3 |
 ��ͬ���칹���У����б����ұ�����һ��ȡ����ֻ��һ�ֵĽṹ���У������������칹����������
��ͬ���칹���У����б����ұ�����һ��ȡ����ֻ��һ�ֵĽṹ���У������������칹����������| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |

| A�� | �����ʽΪC15H22O4 | |
| B�� | һ�������£�l mol���л���������4 mol���������ӳɷ�Ӧ | |
| C�� | 1 mol������������NaOH��Һ��Ӧ���������2 mol NaOH | |
| D�� | �ȿ�����FeCl3��Һ������ɫ��Ӧ���ֿ���ʹ����KMnO4��Һ��ɫ |
| A�� |  | B�� |  | C�� | 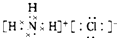 | D�� |  |
| A�� | ����������������������ƽֱ�ӳ��� | |
| B�� | �ƾ��ƵĻ�������õ�ñ���𣬲������촵�� | |
| C�� | �Թ������Թܼ�ȡ�ã��Ҳ���ֱ�Ӽ��� | |
| D�� | ��ȼ�ŵľƾ���ȥ��ȼ��һ���ƾ��� |