��Ŀ����
ij�Ȼ������Ȼ������Ļ�����Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
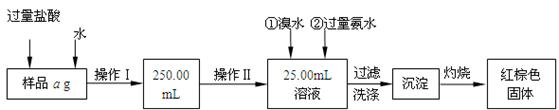
��������������̣��ش��������⣺
��1������I���õ��IJ����������ձ����������⣬��������
��2����д��������ˮ���������ӷ�Ӧ����ʽ
��3������������ȣ���ȴ�����£�����ƽ����������Ϊb1g���ٴμ��Ȳ���ȴ�����³���������Ϊb2g����b1-b2=0.3g�����������Ӧ���еIJ�����
��������������W1g������������Ⱥ������������W2g������Ʒ����Ԫ�ص�����������
��100%
��100%
����ͬѧ����������Բ������·������ⶨ��
��1���ܽ���Ʒ���������ᣬ���������ᣬΪʲô

��2��ѡ��Ļ�ԭ���Ƿ�������
��3�����ζ��õ�c mol/L KMnO4��ҺbmL������Ʒ����Ԫ�ص�����������
��

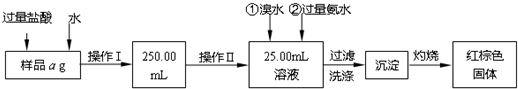
��������������̣��ش��������⣺
��1������I���õ��IJ����������ձ����������⣬��������
250mL����ƿ
250mL����ƿ
����ͷ�ι�
��ͷ�ι�
�������������ƣ���2����д��������ˮ���������ӷ�Ӧ����ʽ
2Fe2++Br2=2Fe3++2Br-
2Fe2++Br2=2Fe3++2Br-
����3������������ȣ���ȴ�����£�����ƽ����������Ϊb1g���ٴμ��Ȳ���ȴ�����³���������Ϊb2g����b1-b2=0.3g�����������Ӧ���еIJ�����
�ٴμ�����ȴ��������ֱ������������С��0.1g
�ٴμ�����ȴ��������ֱ������������С��0.1g
����������������W1g������������Ⱥ������������W2g������Ʒ����Ԫ�ص�����������
| 1120(W2-W1) |
| 160a |
| 1120(W2-W1) |
| 160a |
����ͬѧ����������Բ������·������ⶨ��
��1���ܽ���Ʒ���������ᣬ���������ᣬΪʲô
����������Ժ���KMnO4�ĵζ��и���
����������Ժ���KMnO4�ĵζ��и���

��2��ѡ��Ļ�ԭ���Ƿ�������
��
��
����ǡ�����ԭ���ǣ������������ԭ����������������ᷴӦ����Fe2+��������Ԫ�صIJⶨ
�����������ԭ����������������ᷴӦ����Fe2+��������Ԫ�صIJⶨ
����3�����ζ��õ�c mol/L KMnO4��ҺbmL������Ʒ����Ԫ�ص�����������
| 2.8bc |
| a |
| 2.8bc |
| a |
��������1����������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
��2������Br2���������ԣ�������Fe2+��Ϊ��ʹFe3+��ֳ�������ˮҪ������
��3��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g��������Ԫ�������غ㣬������ɫ���壨 Fe2O3���е���������Ʒ�������������������Ĺ�ʽ�����Ԫ�ص�����������
��1���ܽ���Ʒ�������ᣬ�ø���������õζ�ʱ�����������ӣ�Ӱ��ʵ��ⶨ�����
��2��ij�Ȼ������Ȼ������Ļ�����Ҫ�ⶨ������Ԫ�ص���������������������ԭ�����������ᷴӦ�����������ӣ���������Ԫ�ص����Բⶨ���������
��3�����ݸ�����غ��������ӵ�������ԭ��Ӧ������ϵ���㣮
��2������Br2���������ԣ�������Fe2+��Ϊ��ʹFe3+��ֳ�������ˮҪ������
��3��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g��������Ԫ�������غ㣬������ɫ���壨 Fe2O3���е���������Ʒ�������������������Ĺ�ʽ�����Ԫ�ص�����������
��1���ܽ���Ʒ�������ᣬ�ø���������õζ�ʱ�����������ӣ�Ӱ��ʵ��ⶨ�����
��2��ij�Ȼ������Ȼ������Ļ�����Ҫ�ⶨ������Ԫ�ص���������������������ԭ�����������ᷴӦ�����������ӣ���������Ԫ�ص����Բⶨ���������
��3�����ݸ�����غ��������ӵ�������ԭ��Ӧ������ϵ���㣮
����⣺��1��������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��2����Br2���������ԣ�������Fe2+��2Fe2++Br2=2Fe3++2Br-��Ϊ��ʹFe3+��ֳ�������ˮҪ�������ʴ�Ϊ��2Fe2++Br2=2Fe3++2Br-��
��3��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g������Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3����Ԫ�ص�����Ϊ��W2-W1��g��
����Ʒ����Ԫ�ص�����������
��100%��
�ʴ�Ϊ���ٴμ�����ȴ��������ֱ������������С��0.1g��
��100%��
��1��������ؾ���ǿ�����ԣ��������ᣬ��Һ�е������ӻᱻ�����������ĸ�����أ�����ʵ��ⶨ���ʴ�Ϊ������������Ժ���KMnO4�ĵζ��и��ţ�
��2����ԭ��������������Ϊ�����ֻ�������ᷴӦ�����������������ø�����صζ�������������������������ԭ��������Ԫ�صIJⶨ��
�ʴ�Ϊ���������������ԭ����������������ᷴӦ����Fe2+��������Ԫ�صIJⶨ��
��3�����ݷ�Ӧ5Fe2++MnO4-+8H+=Mn2++5Fe3++4H2O�����ݶ�����ϵ����õ�������Ԫ����������ΪX%��
5Fe2+��5Fe3+��KMnO4
5��56 1
a��X%��
c��b��10-3
��Ԫ�ص�����������X%=
���ʴ�Ϊ��
��
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��2����Br2���������ԣ�������Fe2+��2Fe2++Br2=2Fe3++2Br-��Ϊ��ʹFe3+��ֳ�������ˮҪ�������ʴ�Ϊ��2Fe2++Br2=2Fe3++2Br-��
��3��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g������Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3����Ԫ�ص�����Ϊ��W2-W1��g��
| 112 |
| 160 |
| 1120(W2-W1) |
| 160a |
�ʴ�Ϊ���ٴμ�����ȴ��������ֱ������������С��0.1g��
| 1120(W2-W1) |
| 160a |
��1��������ؾ���ǿ�����ԣ��������ᣬ��Һ�е������ӻᱻ�����������ĸ�����أ�����ʵ��ⶨ���ʴ�Ϊ������������Ժ���KMnO4�ĵζ��и��ţ�
��2����ԭ��������������Ϊ�����ֻ�������ᷴӦ�����������������ø�����صζ�������������������������ԭ��������Ԫ�صIJⶨ��
�ʴ�Ϊ���������������ԭ����������������ᷴӦ����Fe2+��������Ԫ�صIJⶨ��
��3�����ݷ�Ӧ5Fe2++MnO4-+8H+=Mn2++5Fe3++4H2O�����ݶ�����ϵ����õ�������Ԫ����������ΪX%��
5Fe2+��5Fe3+��KMnO4
5��56 1
a��X%��
| 25.00 |
| 250.0 |
��Ԫ�ص�����������X%=
| 2.8bc |
| a |
| 2.8bc |
| a |
������������Ҫ��������Ԫ�ص����������IJⶨ��ʵ�������ʵ�����ݵļ���Ӧ�ã�ͬʱ������ʵ��֪ʶ�ķ����жϣ��ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ