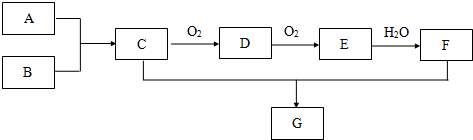
��Ŀ����
15����ҵ�ϳ�����������ʢװ��Ũ���ᣬΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�������1������ȥ�������������������̼�ظ֣�������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ��������Ũ���������������γ���һ�����ܵ������ﱣ��Ĥ����ֹ����������ͭ��Һ�ĽӴ���
��2������ȡ����6.0g����15.0mLŨ�����У����ȣ����Ӧ��õ���ҺX���ռ�������Y��
�ټ�ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+����Ҫȷ�����е�Fe2+������е�ʵ������������������Һ�еμ���ҺX�����Ϻ�ɫ��ȥ������ҺX�к���Fe2+��
����ͬѧȡ336mL����״��������Yͨ��������ˮ�У�������Ӧ��SO2+Br2+2H2O�T2HBr+H2SO4��Ȼ���������BaCl2��Һ�����ʵ�������ø������2.33g���ɴ���֪����Y��SO2���������Ϊ����Ҫ����������̣���Ϊn��BaSO4��=n��SO2��=0.01mol����336mL����״������������ʵ���Ϊ0.015mol������SO2���������Ϊ��$\frac{0.01}{0.015}$��100%=66.7%��
���� ��1��������̼�ظ֣�������Ũ�����У�Ũ�����н�ǿ����������ʹ�����ۻ���
��2����ʵ�����ڼ���Fe2+ʱ��������+2������������ʹ�ữ�ĸ��������ɫ�����飻
�����ɵ�SO2���л�ԭ�ԣ�ͨ��������ˮ�У�����SO2+Br2+2H2O=2HBr+H2SO4��������Ԫ�ص��غ㣬��Ϲ�ϵʽ��SO2��BaSO4���SO2�����������
��� �⣺��1��������̼�ظ֣�������Ũ�����У�Ũ�����н�ǿ����������ʹ�����ۻ���ֹ��Ӧ��һ�����У��ʴ�Ϊ������Ũ���������������γ���һ�����ܵ������ﱣ��Ĥ����ֹ����������ͭ��Һ�ĽӴ���
��2����ʵ�����ڼ���Fe2+ʱ��������+2������������ʹ�ữ�ĸ��������ɫ�����飬���е�ʵ��Ϊ��������������Һ�еμ���ҺX�����Ϻ�ɫ��ȥ������ҺX�к���Fe2+��
�ʴ�Ϊ����������������Һ�еμ���ҺX�����Ϻ�ɫ��ȥ������ҺX�к���Fe2+��
��SO2���л�ԭ�ԣ�ͨ��������ˮ�У�����SO2+Br2+2H2O=2HBr+H2SO4��
n��������壩=$\frac{0.336L}{22.4L/mol}$=0.015mol��
SO2 ��BaSO4
1mol 233g
n 2.33g
n=0.01mol
�� SO2�����������$\frac{0.01}{0.015}$��100%=66.7%��
�ʴ�Ϊ����Ϊn��BaSO4��=n��SO2��=0.01mol����336mL����״������������ʵ���Ϊ0.015mol������SO2���������Ϊ��$\frac{0.01}{0.015}$��100%=66.7%��
���� �����ۺϿ���Ԫ�ػ�����֪ʶ��������貢���ʵ�鷽�������������ֱ��������������Խ�ǿ���漰��Ũ�����ǿ�����ԣ�C��S��Fe���仯��������ʣ���Ŀ�Ѷ��еȣ�

| A�� | �٢� | B�� | �٢ڢ� | C�� | �٢ڢۢ� | D�� | �٢� |
| A�� | NH3 | B�� | SO2 | C�� | HCl | D�� | O2 |
| A�� | ��Ϊ��ɫ���� | B�� | ���Ⱦ��ֽ� | ||
| C�� | ��ˮ��Һ���Լ��� | D�� | ���������ᷴӦ |
Ce4++Fe2+��Fe3++Ce3+
Sn2++2Fe3+��2Fe2++Sn4+��
�ɴ˿���ȷ��Ce4+��Ce3+��Fe3+�������ӵ���������ǿ������˳������
| A�� | Ce4+��Ce3+��Fe3+ | B�� | Ce4+��Fe3+��Ce3+ | C�� | Fe3+��Ce4+��Ce3+ | D�� | Ce3+��Ce4+��Fe3+ |
| A�� | Na2S2����Ԫ�صĻ��ϼ�Ϊ-2 | B�� | NH4Cl�ĵ���ʽ��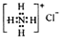 | ||
| C�� | S2-�Ľṹʾ��ͼ��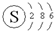 | D�� | COCl2�ĵ���ʽ�� |
| A�� | 2H2������+O2�������T2H2O��������H1 2H2������+O2�������T2H2O��Һ����H2 | |
| B�� | S������+O2�������TSO2��������H1 S���̣�+O2�������TSO2��������H2 | |
| C�� | C���̣�+$\frac{1}{2}$O2 �������TCO��������H1 C���̣�+O2 �������TCO2��������H2 | |
| D�� | H2������+Cl2�������T2HCl��������H1 $\frac{1}{2}$H2������+$\frac{1}{2}$Cl2������=HCl��������H 2 |