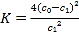��Ŀ����
����Ŀ����ͼ��ʾ����A��B�о�����1mol X��lmol Y����ʼʱA��B�����������a L,����ͬ�¶ȡ����ѹǿ�ʹ������ڵ������£��رջ���K��ʹ�������ж�����������Ӧ�� X��g��+Y��g��![]() 2Z��g��+W��g�� ��H��0����ƽ��ʱ��A�����Ϊ1.4a L������˵���������( )
2Z��g��+W��g�� ��H��0����ƽ��ʱ��A�����Ϊ1.4a L������˵���������( )
A.��Ӧ���ʣ�![]()
B.ƽ��ʱ��ѹǿ��![]()
![]()
C.A������X��ת����Ϊ80%
D.ƽ��ʱY���������A<B
���𰸡�B
��������
A. ��ͼ��֪AΪ��ѹ���̣�BΪ���ݹ��̣����Ҹ÷�ӦΪ�������ķ�Ӧ�����Է�Ӧ������B��ѹǿ����A������ѹǿ�����ʵ�Ӱ���֪����Ӧ����![]() ��A����ȷ��
��A����ȷ��
B. AΪ��ѹ���̣�BΪ���ݹ��̣����Ҹ÷�ӦΪ�������ķ�Ӧ�����Է�Ӧ������B��ѹǿ����A��ƽ��ʱB��ѹǿҲ����A����PB>PA��B�����
C. ����ͬ��ͬ��ʱ�������ѹǿ֮�ȵ��������ʵ���֮�ȣ��ﵽƽ������������ʵ����dz�ʼʱ���ʵ�����1.4������1.4��2mol=2.8mol����������0.8mol�����ݻ�ѧ����ʽ�ļ������֪����
X(g)+Y(g) ![]() 2Z(g)+W(g) ��n
2Z(g)+W(g) ��n
1 1
0.8mol 0.8mol
����ƽ���Ӧ��0.8mol,A������X��ת����Ϊ80%��C����ȷ��
D. AΪ��ѹ���̣�BΪ���ݹ��̣����Ҹ÷�ӦΪ�������ķ�Ӧ�����Է�Ӧ������B��ѹǿ����A������A�еķ�Ӧ������еij̶ȴ���B�еģ�����A��Y���������С��B�еģ���ƽ��ʱY���������A<B��D�����
���Դ�ѡ��B�

����Ŀ�����仯������������;��������ʵ������������±���ʾ��
H2S | S8 | FeS2 | SO2 | SO3 | H2SO4 | |
�۵�/�� | 85.5 | 115.2 | >600���ֽ⣩ | 75.5 | 16.8 | 10.3 |
�е�/�� | 60.3 | 444.6 | 10.0 | 45.0 | 337.0 |
�ش��������⣺
��1����̬Feԭ�Ӽ۲���ӵĵ����Ų�ͼ���������ʽ��Ϊ__________����̬Sԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ_________�Ρ�
��2�����ݼ۲���ӶԻ������ۣ�H2S��SO2��SO3����̬�����У�����ԭ�Ӽ۲���Ӷ�����ͬ���������ӵ���_________��
��3��ͼ��a��ΪS8�Ľṹ�����۵�ͷе�Ҫ�ȶ���������۵�ͷе�ߺܶ࣬��Ҫԭ��Ϊ__________��
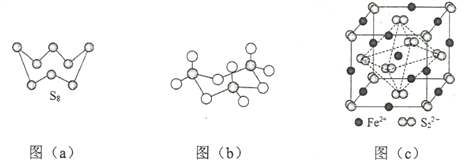
��4����̬���������Ե�������ʽ���ڣ�����ӵ����幹��Ϊ_____�Σ����й��ۼ���������______�֣��������������д�����ͼ��b����ʾ�����۷��ӣ��÷�����Sԭ�ӵ��ӻ��������Ϊ________��
��5��FeS2����ľ�����ͼ��c����ʾ�������߳�Ϊa nm��FeS2���ʽ��ΪM�������ӵ�������ֵΪNA���侧���ܶȵļ������ʽΪ___________g��cm3��������Fe2+λ��![]() ���γɵ�������������ģ�����������ı߳�Ϊ______nm��
���γɵ�������������ģ�����������ı߳�Ϊ______nm��