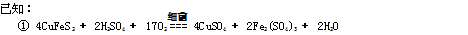��Ŀ����
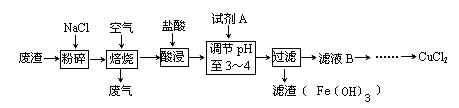
��12�֣���ȸʯ��Ҫ��Cu2(OH)2CO3����������Fe��Si�Ļ����ij�����Կ�ȸʯΪ��Ҫԭ���Ʊ� CuSO4��5H2O�����ײ���G����Ҫ�������£�

��֪�������£�ͨ��������Һ������Զ�ʹFe3+��Fe2+��Cu2 +���ɳ�����pH �ֱ����£�
��ش��������⣺
��1������ͬ�¶�ʱ�ܶȻ�����Դ�С��Ksp[Fe(OH)2]______(�>����<��)Ksp[Cu(OH)2]��
��2����ҺA�еĽ���������Cu2+��Fe2+��Fe3+���Լ�����һ����������Ŀ��������_____________�������ӷ��ţ����ù���ѡ���Լ������Ϊ�����е� ������ţ���
a. Na2O2 b. H2O2 c. Cl2 d. KSCN
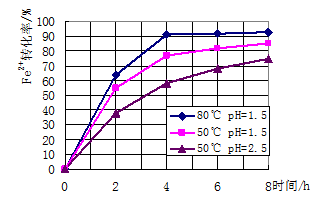
��3��������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ��ȡ�Ĵ�ʩ����Һ�¶ȿ�����_______�棬pH������________������ʱ��Ϊ________Сʱ���ҡ�
��4������ҺB�м����Լ��ڵĻ�ѧʽΪ_______���������������_________��
��5�����ⶨ��ҺA��Fe2+��Ũ�ȣ�����KMnO4����Һ�ζ�����Ӧ��MnO4-����ԭΪMn2+����÷�Ӧ�����ӷ���ʽΪ_____________________________________________��ȡA��Һ20.00 mL����ȥ0.0240 mol/L KMnO4��Һ16.00 mLʱǡ�ôﵽ�ζ��յ㣬��A��Һ��Fe2+Ũ��Ϊ ��

��֪�������£�ͨ��������Һ������Զ�ʹFe3+��Fe2+��Cu2 +���ɳ�����pH �ֱ����£�
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe(OH)3 | 2.2 | 3.2 |
| Fe(OH)2 | 7.6 | 9.6 |
| Cu(OH)2 | 4.7 | 6.4 |
��1������ͬ�¶�ʱ�ܶȻ�����Դ�С��Ksp[Fe(OH)2]______(�>����<��)Ksp[Cu(OH)2]��
��2����ҺA�еĽ���������Cu2+��Fe2+��Fe3+���Լ�����һ����������Ŀ��������_____________�������ӷ��ţ����ù���ѡ���Լ������Ϊ�����е� ������ţ���
a. Na2O2 b. H2O2 c. Cl2 d. KSCN
��3��������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ��ȡ�Ĵ�ʩ����Һ�¶ȿ�����_______�棬pH������________������ʱ��Ϊ________Сʱ���ҡ�

��4������ҺB�м����Լ��ڵĻ�ѧʽΪ_______���������������_________��
��5�����ⶨ��ҺA��Fe2+��Ũ�ȣ�����KMnO4����Һ�ζ�����Ӧ��MnO4-����ԭΪMn2+����÷�Ӧ�����ӷ���ʽΪ_____________________________________________��ȡA��Һ20.00 mL����ȥ0.0240 mol/L KMnO4��Һ16.00 mLʱǡ�ôﵽ�ζ��յ㣬��A��Һ��Fe2+Ũ��Ϊ ��
��12�֣��� >��1�֣� (2) Fe2+����1�֣�b ��1�֣�
��3�� 80 1.5 4 ��3�֣� ��4��CuO��1�֣� ���ˣ�1�֣�
��5��MnO4-+5Fe2++8H+=Mn2++ 5Fe3++4H2O ��2�֣� 0.096mol/L ��2�֣�
��3�� 80 1.5 4 ��3�֣� ��4��CuO��1�֣� ���ˣ�1�֣�
��5��MnO4-+5Fe2++8H+=Mn2++ 5Fe3++4H2O ��2�֣� 0.096mol/L ��2�֣�
��1���ɱ������ݿɿ�����Cu2+��Fe2+��ʼ������PH�ֱ�Ϊ4.7��7.6����Cu2+����OH����Ũ��С����Ksp����ʽ��֪Ksp[Cu(OH)2]��Խ�С��
��2��Ҫ�Ʊ�����ͭ��������ȥ�����Ӻ��������ӡ����ݱ������ݿ�֪Ҫͨ��������ֱ�ӳ�ȥ�������ӣ���ͭ����Ҳ����ͬʱ��ȥ������Ӧ�ð����������������������ӣ�Ȼ����ͨ���������������ӡ���Ϊ������������ȫ������ͭ���ӻ�������Һ�С���ѡ��������������������µ����ʣ�����Ӧ��ѡ����ɫ������˫��ˮ��
��3������ͼ���֪����80��pH����1.5ʱת������ߣ�����Ӧ�ÿ��Ƶ�����������Һ�¶ȿ�����80�棬PH������1.5������ʱ��Ϊ4Сʱ���ҡ�
��4��Ҫ�õ�������������������������Һ��pH��ͬʱ�������������ʣ����Կ�ѡ������ͭ��Ҫ�����Һ������Ҫ���ˡ�
��5���μӷ�Ӧ�ĸ��������0.0240mol��L��0.016L��0.000384mol��������������������ӵķ���ʽΪMnO4��+ 5Fe2+ + 8H+="==" Mn2+ + 5Fe3+ + 4H2O���������ĵ���������Ϊ0.000384mol��5��0.00192mol������Ũ��Ϊ
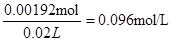

��ϰ��ϵ�д�
�����Ŀ