��Ŀ����
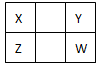
��10�֣�X ��Y�� Z�� WΪ���ֳ����Ķ�����Ԫ�ء�����YԪ��ԭ�Ӻ�������������������Ӳ�����3�������������ڱ��е����λ������ͼ��ʾ��
��ش��������⣺
��1��Wλ�����ڱ��е�______________���ڣ���___________�塣
��2��X������Թ���+1�������ӣ������ʽ�� ��Y����̬�⻯����ȶ���
��Z����̬�⻯����ȶ��� ���ǿ��������������
��3��X������������ˮ���������⻯���ܻ�������M, M��ˮ��Һ�����Ե�ԭ����
_____________ __�������ӷ���ʽ��ʾ����
��4����Y��Z�����һ����̬������Q��Q����W�ĵ����ڳ�ʪ�����з�Ӧ����Ӧ�Ļ�ѧ����ʽ��
 ��
��
����һ�������£�������Q��Y�ĵ��ʷ�Ӧ��ƽ��ʱ��������̬���ʣ���Ӧʱ ��ÿת��4mol���ӷ���190.0kJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��ÿת��4mol���ӷ���190.0kJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
| X | Y | |
| | Z | W |
��1��Wλ�����ڱ��е�______________���ڣ���___________�塣
��2��X������Թ���+1�������ӣ������ʽ�� ��Y����̬�⻯����ȶ���
��Z����̬�⻯����ȶ��� ���ǿ��������������
��3��X������������ˮ���������⻯���ܻ�������M, M��ˮ��Һ�����Ե�ԭ����
_____________ __�������ӷ���ʽ��ʾ����
��4����Y��Z�����һ����̬������Q��Q����W�ĵ����ڳ�ʪ�����з�Ӧ����Ӧ�Ļ�ѧ����ʽ��
 ��
������һ�������£�������Q��Y�ĵ��ʷ�Ӧ��ƽ��ʱ��������̬���ʣ���Ӧʱ
 ��ÿת��4mol���ӷ���190.0kJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��ÿת��4mol���ӷ���190.0kJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ������10�֣�
(1) ��������VIIA
��2��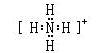 ��ǿ
��ǿ
��3�� NH4+ +H2O NH
NH 3��H2O + H+
3��H2O + H+
��4��SO2 + Cl2 + 2H2O =" 2HCl" + H2SO4
2SO2��g��+ O2��g�� 2SO3��g����H =" -190.0" kJ��mol��1
2SO3��g����H =" -190.0" kJ��mol��1
(1) ��������VIIA
��2��
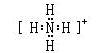 ��ǿ
��ǿ��3�� NH4+ +H2O
 NH
NH 3��H2O + H+
3��H2O + H+��4��SO2 + Cl2 + 2H2O =" 2HCl" + H2SO4
2SO2��g��+ O2��g��
 2SO3��g����H =" -190.0" kJ��mol��1
2SO3��g����H =" -190.0" kJ��mol��1��

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ
 2s
2s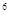 3s
3s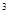 4s
4s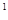 �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��Ԫ�ط���Ϊ ��������������Ӧ��ˮ��������Ա�̼�� ����
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��Ԫ�ط���Ϊ ��������������Ӧ��ˮ��������Ա�̼�� ���� ��ǿ������������
��ǿ������������ ���죬��������O3���ɣ�����˵����ȷ���ǣ�
���죬��������O3���ɣ�����˵����ȷ���ǣ� 3�ת���������仯
3�ת���������仯