��Ŀ����
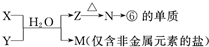
X��Y��Z��M��N��K���ɶ�����Ԫ�ع��ɵ���������X��Y��Z�������ӣ�M��N�����Է��ӣ�K�������ӣ����Ǿ������нṹ�ص�����ʣ�
�����Ǻ��������������ͬ�� ��N����M�У������̪����Һ��죻
��X��N����A��C��Ԫ����ɣ�X��Y������������ȣ�
��Y��K����A��B��Ԫ����ɣ�Y��������������K��������
��ZΪ�������ӣ������Ӱ뾶�����������ͬ���Ӳ�ṹ�����а뾶��С�ġ�
����������Ϣ����ش��������⣺
��1��Y�Ļ�ѧʽΪ ��X�ĵ���ʽΪ ��
��2���ԱȽ�M��N���ȶ��ԣ�M N���>������<������=����
��3������X�ķ����� ��
��4����A��B��C����Ԫ����ɵ������У��������ӻ�������� �����ڹ��ۻ�������� ������дһ�����ʵĻ�ѧʽ��
��5�������������е����ֿ���������γ�һ�ָ��Σ�����ε�Ũ��Һ����μ���0.1mol/L����������Һ����������������Һ�ļ��룬���������Ĺ�ϵ����ͼ����ø��εĻ�ѧʽΪ ��
1��H3O+��2�֣���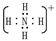 ��2�֣�
��2�֣�
��2��>��2�֣�
��3�����Թ�ȡ��X����Һ��������������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壨2�֣�
��4��NH4NO3�ȣ�2�֣���HNO3�ȣ�2�֣�
��5��(NH4)3 Al(SO4)3��3(NH4)2 SO4�qAl2(SO4)3��3�֣�
�������� ��N����M�пɳ����ж�MΪH2O��������ܼ��������ɢ�֪����10���ӣ�N��ˮ��Һ�Լ��ԣ�����NΪNH3����ѧ��ѧ��Ψһ�ļ������壩���ɢ���A��B����Ԫ���γɵ�����������������2����YΪH3O����KΪOH����A��BΪH��O��Ԫ�أ��ɢ�֪��XΪNH4������CΪNԪ�أ� ZΪ�������ӣ������Ӱ뾶�����������ͬ���Ӳ�ṹ�����а뾶��С�ģ���ZΪ�����ӣ���
��1��YΪH3O����XΪNH4��������ʽΪ���ʴ�Ϊ��H3O����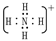 ��
��
��2��MΪH2O��NΪNH3�����ڷǽ����ԣ�O��N��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ������ȶ��ԣ�H2O��NH3���ʴ�Ϊ������
��3�����飬�ɸ���NH4����OH����Ӧ���ɼ�������NH3����ʹʪ��ĺ�ɫʯ����ֽ�������м��飬�ʴ�Ϊ�����Թ�ȡ��X����Һ��������������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壻
��4��A��BΪH��OԪ�أ�CΪNԪ�أ��γɵ����ӻ�������NH4NO3��NH4NO2�ȣ����ۻ�������HNO3��HNO2�ȣ��ʴ�Ϊ��NH4NO3�ȣ�HNO3�ȣ�
��5�������ɰ�ɫ������˵������Al3������ʼ������Al3��+3OH��=Al��OH��3����Ȼ��������������䣬��Ӧ������NH4��+OH��=NH3��H2O���������Al��OH��3+OH��=AlO2��+2H2O������ͼ��������֪��n��NH4������n��Al3����=3��1����ø��εĻ�ѧʽΪ��NH4��3 Al��SO4��3��3��NH4��2 SO4�qAl2��SO4��3��
�ʴ�Ϊ����NH4��3 Al��SO4��3��3��NH4��2 SO4�qAl2��SO4��3��

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�

 ��
��

 4Al+3O2��
4Al+3O2��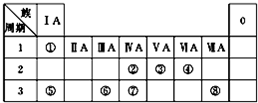 ��ͼΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
��ͼΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺