��Ŀ����
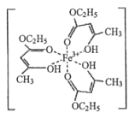
����Ŀ��(1)��³ʿ���Ļ�ѧʽ��KFe[Fe(CN)6]�������ʵĻ�ѧ�������Ӽ������ۼ���_____��
(2)KOCN�����Ӿ��壻̼ԭ�Ӳ�ȡsp�ӻ���1mol�������к��е�������ĿΪ____��
(3)H2O2��������Һ�壬�е�ϸ�(150��)������Ҫԭ����____��
(4)�ǽ���Ԫ��![]() �ĵ�һ�����ܴ���
�ĵ�һ�����ܴ���![]() �ĵ�һ�����ܣ�ԭ����______��
�ĵ�һ�����ܣ�ԭ����______��
(5)V2O5����NaOH��Һ���ɵõ�������(Na3VO4)�����������ӵ����幹��Ϊ________��
(6)��֪ʳ�ε��ܶ�Ϊ�� g��cm��3����Ħ������ΪM g��mol��1�������ӵ�����ΪNA������ʳ�ξ��徧��������_______cm��
(7)1 mol SiO2�����к�________ mol Si��O����
(8)1 mol NH4BF4����________ mol�����
(9)�������ʯ��MgO��CaCl2���ɱ�5�־�����۵��ɸߵ��͵�˳��Ϊ��_____________��
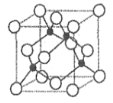
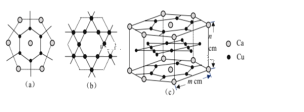
(10)���������ṹ�Ľ����磺Na��K��Fe�ľ����Ŀռ�ռ���ʱ���ʽΪ________(����)��
���𰸡���λ�� 2NA ˫��ˮ���Ӽ������� ��̬Nԭ�ӵ�2p������ڰ������״̬�����������ȶ������N�ĵ�һ�����������O�ĸ��� �������� 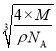 4 2 ���ʯ��MgO��CaCl2�������ɱ�
4 2 ���ʯ��MgO��CaCl2�������ɱ� ![]()
��������
(1) KFe[Fe(CN)6]��������������г����Ӽ������ۼ��⣬��������λ����
(2)�����֪��OCN-�е�C���õ���sp�ӻ�����ˣ���C��Oԭ��֮���γɵ�������C��N֮���γ����������ԣ�1��OCN-����2���Ҽ���2���м���1molKOCN�к���2NA���м���
(3)��������ĽṹΪH-O-O-H����˹���������Ӽ���γ������ʹ������нϸߵķе㣻
(4)��̬Nԭ�ӵļ۵����Ų�ʽΪ2s22p3����̬Oԭ�ӵļ۵����Ų�ʽΪ2s22p4����̬Nԭ�ӵ�2p������ڰ������״̬�����������ȶ������N�ĵ�һ�����������O�ĸ��ߣ�
(5)��̬Vԭ�ӵļ۵����Ų�ʽΪ3d34s2����������![]() �м۲���Ӷ���Ϊ4�������µ��Ӷԣ�����
�м۲���Ӷ���Ϊ4�������µ��Ӷԣ�����![]() �Ĺ���Ϊ�������壻
�Ĺ���Ϊ�������壻
(6)ʳ����Ҫ�ɷּ�NaCl��NaCl�ľ����к���4��Na+��4��Cl-������侧������Ϊ��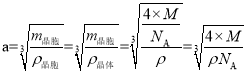 ��
��
(7)SiO2����Ļ����ṹ��Ԫ��![]() �����壬ÿ��Siԭ����Χ�����4��O�����SiO2��Siԭ�Ӹ�����Si-O��������Ϊ1:4������1molSiO2�к���4molSi-O����
�����壬ÿ��Siԭ����Χ�����4��O�����SiO2��Siԭ�Ӹ�����Si-O��������Ϊ1:4������1molSiO2�к���4molSi-O����
(8)![]() �к���1����λ����
�к���1����λ����![]() ��Ҳ����1����λ�������1mol
��Ҳ����1����λ�������1mol![]() �к���2mol��λ����
�к���2mol��λ����
(9)�����ڷ��Ӿ��壬��Ҫ��ˮ����ͨ������γɣ����ʯ����ԭ�Ӿ��壬��̼ԭ��ͨ�����ۼ��γɣ�MgO�������Ӿ��壬��Mg2+��O2-ͨ�����Ӽ��γɣ�CaCl2�������Ӿ��壬��Ca2+��Cl-ͨ�����Ӽ��γɣ��ɱ����ڷ��Ӿ��壬��CO2����ͨ�����»����γɣ����־����۵��ɸߵ��͵�˳��Ϊ�����ʯ��MgO��CaCl2�������ɱ���
(10)���������ṹ�Ľ����������߳�a��ԭ�Ӱ뾶�Ĺ�ϵΪ��![]() ��һ�������к���
��һ�������к���![]() ��ԭ�ӣ���˿ռ�������Ϊ��
��ԭ�ӣ���˿ռ�������Ϊ��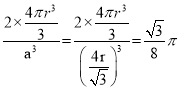 ��
��