��Ŀ����
��15�֣��������Ļ������ڹ�ҵ�������ճ������ж��й㷺����;����ش��������⣺
��1����Ԫ�������ڱ��е�λ���� ��
��2��д��Fe��ϡ���ᷴӦ�����ӷ���ʽ ��
��3����֪��Fe2O3��s��+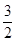 C��s��=
C��s��=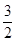 CO2��g��+2Fe��s������H=+234.1kJ/mol
CO2��g��+2Fe��s������H=+234.1kJ/mol
C��s��+O2��g��=CO2��g��; ��H=-393.5kJ/mol��
��д��Fe��s����O2��g����Ӧ����Fe2O3��s�����Ȼ�ѧ����ʽ ��
��4����һ�������£�������������һ����̼�������з�Ӧ��
Fe2O3��s��+3CO��g��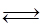 2Fe��s��+3CO2��g��;��ҵ����16 t Fe2O3��200m3�������з�Ӧ��lСʱ����Fe2O3��ת����Ϊ50%�������ʱ����CO����������Ϊ mol/��L��h����
2Fe��s��+3CO2��g��;��ҵ����16 t Fe2O3��200m3�������з�Ӧ��lСʱ����Fe2O3��ת����Ϊ50%�������ʱ����CO����������Ϊ mol/��L��h����
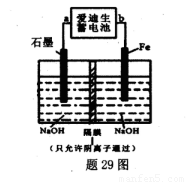
��5�����������صķ�ӦʽΪ��Fe+NiO2+2H2O Fe��OH��2+Ni��OH��2���������ƣ� Na2FeO4����һ��������ˮ������������29ͼװ�ÿ�����ȡ�����������ƣ�
Fe��OH��2+Ni��OH��2���������ƣ� Na2FeO4����һ��������ˮ������������29ͼװ�ÿ�����ȡ�����������ƣ�
�ٴ�װ���а��������صĸ�����____���a����"b������
��д�������ĵ缫��Ӧʽ________��
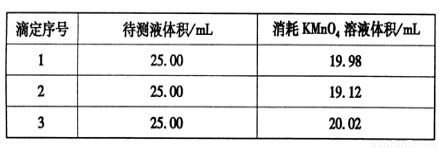
�۵��Ƶ�132��8g��������ʱ�����������ٵ������ӵ����ʵ���Ϊ ��
��1���������ڣ����壨2�֣�
��2��Fe��2H��= Fe2����H2����2�֣�
��3��2Fe(s)��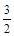 O2(g) = Fe2O3(s)
��H=��824.4kJ/mol��3�֣�
O2(g) = Fe2O3(s)
��H=��824.4kJ/mol��3�֣�
��4��0.75��2�֣�
��5����a��1�֣�
��Fe��6e����8OH����FeO42����4H2O��3�֣� ��0.8mol��2�֣�
����������1������ԭ��������26��λ�ڵ������ڣ����塣
��2������ϡ���ᷴӦ������������������������ʽΪFe��2H��= Fe2����H2����
��3�������˹���ɵ�Ӧ�á�������֪��Ӧ��֪�ڡ�2/3���ټ��õ�2Fe(s)��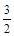 O2(g) = Fe2O3(s) �����Է�Ӧ���ǣ�393.5kJ/mol��3/2��234.1kJ/mol����824.4kJ/mol.
O2(g) = Fe2O3(s) �����Է�Ӧ���ǣ�393.5kJ/mol��3/2��234.1kJ/mol����824.4kJ/mol.
��4��lСʱ����Fe2O3��ת����Ϊ50%����������������8t���������CO��
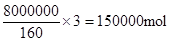 �������䷴Ӧ������
�������䷴Ӧ������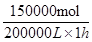 ��0.75mol/��L��h����
��0.75mol/��L��h����
��5������ȡ�������ƣ�����Ӧ��������������a�Ǹ�����b��������
�ڵ���������ʧȥ���ӣ��������������Ǹ������ƿ�֪�������缫��ӦʽΪFe��6e����8OH����FeO42����4H2O��
��132��8g����������132.8g��166g/mol��0.8mol�����Ը��ݷ���ʽ2H2O��Fe��2OH�� FeO42����3H2����֪������1.6molOH������ͬʱ����0.8molFeO42�������Խ�����������0.8mol��
FeO42����3H2����֪������1.6molOH������ͬʱ����0.8molFeO42�������Խ�����������0.8mol��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д� �������Ļ������ڹ�ҵ�������ճ������ж��й㷺����;����ش��������⣺
�������Ļ������ڹ�ҵ�������ճ������ж��й㷺����;����ش��������⣺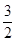 C��s��=
C��s��= 2Fe��s��+3CO2��g��;��ҵ����16 t Fe2O3��200m3�������з�Ӧ��lСʱ����Fe2O3��ת����Ϊ50%�������ʱ����CO����������Ϊ mol/��L��h����
2Fe��s��+3CO2��g��;��ҵ����16 t Fe2O3��200m3�������з�Ӧ��lСʱ����Fe2O3��ת����Ϊ50%�������ʱ����CO����������Ϊ mol/��L��h���� Fe��OH��2+Ni��OH��2���������ƣ� Na2FeO4����һ��������ˮ������������29ͼװ�ÿ�����ȡ�����������ƣ�
Fe��OH��2+Ni��OH��2���������ƣ� Na2FeO4����һ��������ˮ������������29ͼװ�ÿ�����ȡ�����������ƣ�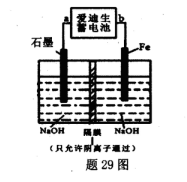
 Fe��OH��2+Ni��OH��2�����������ƣ�Na2FeO4����һ�����;�ˮ����������װ�ÿ�����ȡ�����������ƣ�
Fe��OH��2+Ni��OH��2�����������ƣ�Na2FeO4����һ�����;�ˮ����������װ�ÿ�����ȡ�����������ƣ�