��Ŀ����
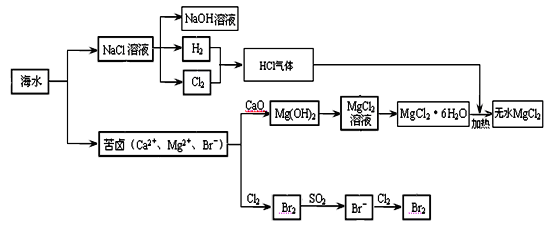
�����Ǿ�Ļ�ѧ��Դ���⡣�����Ǻ�ˮ��ѧ��Դ�ۺ����õIJ�������ͼ��
�ش�
��1���ɺ�ˮɹ�ƵĴ����к���Ca2+��Mg2+��SO42�������ӣ�Ϊ�˳�ȥ��Щ���ӣ���Ҫ���μ����Թ�����NaOH��BaCl2��__ ___�����Լ���ѧʽ����Ȼ��__ ___����������ƣ�������Һ���ټ����� �����Լ���������������Һ���õ����Ρ�
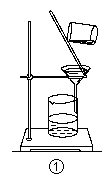
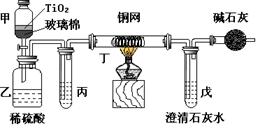
��2��ijͬѧ��ʵ����ģ���ȼҵ������ԭ����ⱥ��ʳ��ˮ���ò�����պŨ��ˮ�����������������壬���ֲ����������̡��������ɵ������� �����̵���Ҫ�ɷ��� ��
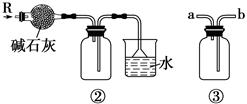
��3����ȡMgCl2�Ĺ������漰��Ӧ��MgCl2��6H2O MgCl2 + 6H2O���÷�ӦҪ��HCl�����н��У�ԭ���� ��
MgCl2 + 6H2O���÷�ӦҪ��HCl�����н��У�ԭ���� ��
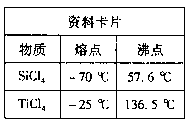
��4����±��ͨ��Cl2�û���Br2����������SO2����ת��ΪBr����������Σ��Դﵽ�������Ŀ�ġ��ɺ�ˮ��������еķ�Ӧ�ɵó�Cl����SO2��Br����ԭ����ǿ������˳����_________��

�ش�
��1���ɺ�ˮɹ�ƵĴ����к���Ca2+��Mg2+��SO42�������ӣ�Ϊ�˳�ȥ��Щ���ӣ���Ҫ���μ����Թ�����NaOH��BaCl2��__ ___�����Լ���ѧʽ����Ȼ��__ ___����������ƣ�������Һ���ټ����� �����Լ���������������Һ���õ����Ρ�
��2��ijͬѧ��ʵ����ģ���ȼҵ������ԭ����ⱥ��ʳ��ˮ���ò�����պŨ��ˮ�����������������壬���ֲ����������̡��������ɵ������� �����̵���Ҫ�ɷ��� ��
��3����ȡMgCl2�Ĺ������漰��Ӧ��MgCl2��6H2O
 MgCl2 + 6H2O���÷�ӦҪ��HCl�����н��У�ԭ���� ��
MgCl2 + 6H2O���÷�ӦҪ��HCl�����н��У�ԭ���� ����4����±��ͨ��Cl2�û���Br2����������SO2����ת��ΪBr����������Σ��Դﵽ�������Ŀ�ġ��ɺ�ˮ��������еķ�Ӧ�ɵó�Cl����SO2��Br����ԭ����ǿ������˳����_________��
��1��Na2CO3�����ˡ������HCl����1�֣� ��2��Cl2��������NH4Cl����1�֣�
��3��MgCl2����ˮ�⣬MgCl2 + 2H2O Mg(OH)2 + 2HCl ����HCl �����У���������MgCl2ˮ�⣬ͬʱ����ˮ�֡���2�֣�����Ҫ���1�֣� ��4��SO2��Br-��Cl-��2�֣�
Mg(OH)2 + 2HCl ����HCl �����У���������MgCl2ˮ�⣬ͬʱ����ˮ�֡���2�֣�����Ҫ���1�֣� ��4��SO2��Br-��Cl-��2�֣�
��3��MgCl2����ˮ�⣬MgCl2 + 2H2O
 Mg(OH)2 + 2HCl ����HCl �����У���������MgCl2ˮ�⣬ͬʱ����ˮ�֡���2�֣�����Ҫ���1�֣� ��4��SO2��Br-��Cl-��2�֣�
Mg(OH)2 + 2HCl ����HCl �����У���������MgCl2ˮ�⣬ͬʱ����ˮ�֡���2�֣�����Ҫ���1�֣� ��4��SO2��Br-��Cl-��2�֣������������1��Ca2����̼���Ƴ�ȥ��Mg2�����������Ƴ�ȥ��SO42�����Ȼ�����ȥ�������������ữ�������ڹ������Ȼ���Ҫ��̼��������������̼���Ʊ�������Ȼ����ĺ��棬���������ƿ����������������Ϊ�˳�ȥ��Щ���ӣ���Ҫ���μ����Թ�����NaOH��BaCl2��Na2CO3��Ȼ����ˡ�����Һ���ټ��������ᣬ����������Һ���õ����Ρ�
��2���ò�����պŨ��ˮ�����������������壬���ֲ����������̣��ð���Ӧ�����Ȼ�粒����������˵����Ӧ�����Ȼ������ɣ������������������������������ǿ�����ԣ��ܰѰ����������ɵ������Ȼ��⣬Ȼ���Ȼ����백����Ӧ���������̡�
��3��MgCl2��ǿ�������Σ�þ��������ˮ�⣬MgCl2 + 2H2O
 Mg(OH)2 + 2HCl��ˮ�����ȣ����ȴٽ�ˮ�⣬������HCl �����У���������MgCl2ˮ�⣬ͬʱ����ˮ�֣��Ӷ��õ��Ȼ�þ���塣
Mg(OH)2 + 2HCl��ˮ�����ȣ����ȴٽ�ˮ�⣬������HCl �����У���������MgCl2ˮ�⣬ͬʱ����ˮ�֣��Ӷ��õ��Ȼ�þ���塣��4����������ԭ��Ӧ�л�ԭ���Ļ�ԭ��ǿ�ڻ�ԭ����Ļ�ԭ�ԣ����Ը��ݿ�±��ͨ��Cl2�û���Br2��֪����ԭ��Ӧ����������ǿ�������ӡ���������SO2����ת��ΪBr������˵���ڷ�Ӧ�е������SO2��������������廯�⣬����SO2�Ļ�ԭ��ǿ�������ӵĻ�ԭ�ԣ��ɴ˿ɵó�Cl����SO2��Br����ԭ����ǿ������˳����SO2��Br-��Cl-��

��ϰ��ϵ�д�
 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
�����Ŀ