题目内容
蛇纹石矿可以看作由MgO、Fe2O3、Al2O3、SiO2组成,由蛇纹石制取碱式碳酸镁的实验步骤如下:

(1)蛇纹石矿加盐酸溶解后,溶液里除了Mg2+外,还含有的金属离子是 。
(2)进行Ⅰ操作时,控制溶液的pH=7~8(有关氢氧化物沉淀的pH见下表),Ca(OH)2不能过量,若Ca(OH)2过量可能会导致 溶解,产生 沉淀。
(3)从沉淀混合物A中提取红色氧化物作为颜料,先向沉淀物A中加入 (填加入物质的化学式),然后 (依此填写实验操作名称)。物质循环使用,能节约资源。上述实验中,可以循环使用的物质是 (填写物质的化学式)。
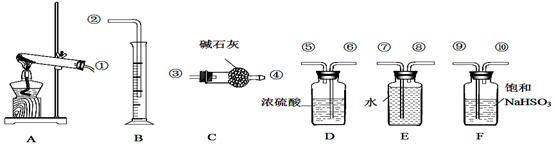
(4)现设计实验,确定产品aMgCO3·bMg(OH)2·cH2O中a、b、c的值。请写出下列实验步骤中所需要测定的项目(可用试剂:浓硫酸、碱石灰、氢氧化钠溶液、澄清石灰水):①样品称量,②高温分解,③ ,④ ,⑤MgO称量。
(5)从下列仪器(装有必要的试剂)中选择完成上述实验所必需的仪器,连接一套装置 (选择仪器代号,可重复使用,用“A→B→……→”表示)

(6)18.2g产品完全分解后,产生6.6gCO2和8.0gMgO,由此可知,产品的化学式中a= ,b= ,c= 。

(1)蛇纹石矿加盐酸溶解后,溶液里除了Mg2+外,还含有的金属离子是 。
(2)进行Ⅰ操作时,控制溶液的pH=7~8(有关氢氧化物沉淀的pH见下表),Ca(OH)2不能过量,若Ca(OH)2过量可能会导致 溶解,产生 沉淀。
| 氢氧化物 | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| 开始沉淀pH | 1.5 | 3.3 | 9.4 |
(3)从沉淀混合物A中提取红色氧化物作为颜料,先向沉淀物A中加入 (填加入物质的化学式),然后 (依此填写实验操作名称)。物质循环使用,能节约资源。上述实验中,可以循环使用的物质是 (填写物质的化学式)。
(4)现设计实验,确定产品aMgCO3·bMg(OH)2·cH2O中a、b、c的值。请写出下列实验步骤中所需要测定的项目(可用试剂:浓硫酸、碱石灰、氢氧化钠溶液、澄清石灰水):①样品称量,②高温分解,③ ,④ ,⑤MgO称量。
(5)从下列仪器(装有必要的试剂)中选择完成上述实验所必需的仪器,连接一套装置 (选择仪器代号,可重复使用,用“A→B→……→”表示)

(6)18.2g产品完全分解后,产生6.6gCO2和8.0gMgO,由此可知,产品的化学式中a= ,b= ,c= 。
(1)Fe3+、Al3+
(2)Al(OH)3、Mg(OH)2;
(3)NaOH,过滤、洗涤、灼烧,CO2。
(4)测出蒸气的质量,测出二氧化碳的质量。
(5)A→C→D→D
(6)3 1 3
(2)Al(OH)3、Mg(OH)2;
(3)NaOH,过滤、洗涤、灼烧,CO2。
(4)测出蒸气的质量,测出二氧化碳的质量。
(5)A→C→D→D
(6)3 1 3
试题分析:(1)蛇纹石矿可以看做MgO、Fe2O3、Al2O3、SiO2组成,蛇纹石加盐酸溶解后,MgO、Fe2O3、Al2O3和HCl反应溶解,而SiO2和HCl不反应,不能溶解。
(2)当氢氧化钙过量时,溶液碱性增强,Al(OH)3会溶解,从氢氧化物沉淀的pH表中可看出,Mg(OH)2在pH为9.4时开始沉淀,所以碱性增强Mg(OH)2会沉淀。
(3)红色氧化物为Fe2O3,应先将其中含有的少量Al(OH)3除去,除去Al(OH)3的方法是利用它能溶于强碱的性质,此过程中CO2是可以重复使用的。
(4)确定产品aMgCO3?bMg(OH)2?cH2O中a、b、c的值,需要测定的数据是①样品质量;②MgO质量;③生成CO2的质量(或体积);④生成水的质量。
(5)A为样品反应装置,加热后产生水蒸汽和二氧化碳气体,为测定二者质量,应首先连接C装置,测定水的质量,故连接C;继续测定二氧化碳,此时应选用D,由于B中溶液含有水,与C连接易被C吸收,产生误差。为防止空气中的二氧化碳与水蒸汽被D吸收产生误差,故应连续连接两个D装置。故答案为A→C→D→D。
(6)m(样品)=18.2g,m(CO2)=6.6g,m(MgO)=8.0g,碱式碳酸镁分解:aMgCO3?bMg(OH)2?cH2O
 (a+b)MgO+aCO2↑+(b+c)H2O↑,根据质量守恒得:m(H2O)=18.2g-6.6g-8.0g=3.6g,则m(MgO)═0.2mol,n(CO2)═0.15mol,n(H2O)═0.2mol,得:a:b:c=0.15:0.05:0.15=3:1:3。
(a+b)MgO+aCO2↑+(b+c)H2O↑,根据质量守恒得:m(H2O)=18.2g-6.6g-8.0g=3.6g,则m(MgO)═0.2mol,n(CO2)═0.15mol,n(H2O)═0.2mol,得:a:b:c=0.15:0.05:0.15=3:1:3。
练习册系列答案
 举一反三单元同步过关卷系列答案
举一反三单元同步过关卷系列答案
相关题目

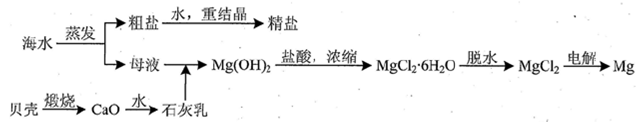


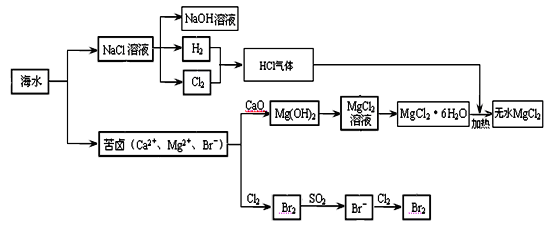
 MgCl2 + 6H2O,该反应要在HCl气流中进行,原因是 。
MgCl2 + 6H2O,该反应要在HCl气流中进行,原因是 。
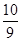 ,其平衡常数表达式为 最终所得气体的平均相对分子质量为__________(保留一位小数)
,其平衡常数表达式为 最终所得气体的平均相对分子质量为__________(保留一位小数)



