��Ŀ����
1����1�����ͱ���ͬϵ�������ɷ���ͨʽCnH2n-6��n��6����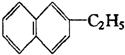 ��������ͬϵ�����������ͬϵ��������ͨʽΪCnH2n-12 ��n��10����
��������ͬϵ�����������ͬϵ��������ͨʽΪCnH2n-12 ��n��10������2����һ�������£�ijЩ�����������ӿ����������мӳɷ�Ӧ�����磺CH��CH+CH��CH��CH��CһCH=CH2���л���Ľṹ��ʽΪ��CH2=C��CH3��һCH2һC��CH3��3�������ɲ��������ҵ�����������һ�������������ӳɶ��õ��ģ��ڴ˷�Ӧ�г����ɼ��⣬��ͬʱ�õ���һ�ֲ���������л���������̼����Ϊ5��̼ԭ�ӣ����Ǽ�ͬ���칹�壮
���ҵĽṹ��ʽΪ
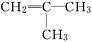 �ڱ��Ľṹ��ʽΪ
�ڱ��Ľṹ��ʽΪ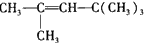 ��
��
���� ��1����ϵ�з��ӽṹ����һ��������������7�������Ͷȣ��ݴ˽�ɣ�
��2�������л���Ľṹ��ʽΪCH2=C��CH3��һCH2һC��CH3��3����Ӧԭ��CH��CH+CH��CH��CH��CһCH=CH2�����ƶϳ��л����ҵĽṹ��ʽ��Ȼ�����ͬ���칹����ص㡢��Ӧԭ�������Ҫ��д�����Ľṹ��ʽ��
��� �⣺��1����ϵ�з��ӽṹ����һ��������������7�������Ͷȣ�����ԭ�Ӹ���Ϊ��2n+2-14=2n-12������ͬϵ���ͨʽΪCnH2n-12������ע���������̼ԭ��������Ϊ10���ʣ�n��10���ʴ�Ϊ��CnH2n-12 ��n��10����
��2������Ŀ�ṩ����Ϣ���Ľṹ��ʽCH2=C��CH3��һCH2һC��CH3��3��֪���л��������2���Ӳ��������ҷ�����Ϣ�еļӳɷ�Ӧ�õ��ģ����ҵĽṹ��ʽΪ��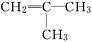 ��
��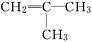 ���Ӱ����·�ʽ��
���Ӱ����·�ʽ�� ���������ӳɵõ���һ�ֲ������ߵġ��̼����Ϊ5��̼ԭ�ӵ��л������
���������ӳɵõ���һ�ֲ������ߵġ��̼����Ϊ5��̼ԭ�ӵ��л������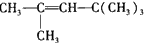 ��
��
�ʴ�Ϊ��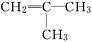 ��
��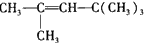 ��
��
���� ���⿼���л����ͨʽ�Լ��ṹ��ʽ���жϣ�����Ϊ�е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ�����������������ͽ��ⷽ����ָ����ѵ��������Ĺؼ��Ǹ�����֪����Ϣ�ó���Ӧ��ԭ����Ȼ�������⣬������ü��ɣ���������������ѧ���Ĵ���˼ά����������˼ά������

��1��N2��O2��H2�֮����Է������Ϸ�Ӧ����֪��Ӧ���Ȼ�ѧ����ʽ���£�
N2��g��+O2��g���T2NO��g����H=+180.5kJ•mol-1��
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ•mol-1��
N2��g��+3H2��g���T2NH3��g����H=-92.4kJ•mol-1��
�Ĵ�������Ӧ���Ȼ�ѧ����ʽΪ4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905kJ/mol��
��2������β��������һ����Ӧԭ��Ϊ��2NO��g��+2CO��g��?N2��g��+2CO2��g����H��0��һ���¶��£���2.8mol NO��2.4mol COͨ��̶��ݻ�Ϊ2L���ܱ������У���Ӧ�����в������ʵ����ʵ����仯��ͼ1��ʾ��

��NO��ƽ��ת����Ϊ28.57%��0��20minƽ����Ӧ����v��NO��Ϊ0.02mol/��L•min����25minʱ�������ַ�Ӧ�¶Ȳ��䣬���������г���CO��N2��0.8mol����ѧƽ�⽫�����ƶ�������������ҡ���������
����ͼ2��ֻ�ı�ijһ��Ӧ����X����Ӧ��ԭƽ��I�ﵽ��ƽ�����Y�ı仯���������ʾ������˵����ȷ����b ������ĸ���ţ���
| ����X | ����Y | |
| a | ѹǿ | ��Ӧ��ƽ�ⳣ�� |
| b | �¶� | CO��ƽ��Ũ�� |
| c | �¶� | N2��������� |
| d | ���� | NO��ƽ��ת���� |
a���ŵ�����У��������Һ��pH���ֲ���
b����Һ�е�NH4ClŨ������Cl-����Ũ�Ȳ���
c��ÿת��6.02��1023�����ӣ����б�״����11.2L�缫��Ӧ�ﱻ����
d��Ϊ���ַŵ�Ч�������ʹ��һ��ʱ��������������Һ��
| A�� | 0.5 mol 1��3-����ϩ�����к���C=C˫����Ϊ NA | |
| B�� | 1 mol�ǻ���-OH�������ĵ�������Ϊ9NA | |
| C�� | 14g��ϩ����ϩ�Ļ������������ԭ������Ϊ3NA | |
| D�� | ��״���£�1L������ȫȼ�������ɵ���̬����ķ�����Ϊ7/22.4NA |
| A�� | 2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2�� | B�� | 4Fe��OH��2+O2+2H2O=4Fe��OH��3 | ||
| C�� | 2F2+2H2O=4HF+O2�� | D�� | 2Na+2H2O=2NaOH+H2�� |
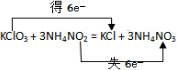
| A�� | Cl2������ԭ��Ӧ | |
| B�� | �������뻹ԭ�������ʵ���֮��Ϊ1��2 | |
| C�� | ����Ӧ������3molH2O����ת�Ƶ���2mol | |
| D�� | ���������ø÷�Ӧ���Խ���ѧ��ת��Ϊ���� |
| A�� | �÷�Һ©�����ձ�����ˮ�����������Ļ���� | |
| B�� | �ô����������ռ� | |
| C�� | �ò�����պ�����������ʵ�Ũ��Һ����ɫ��Ӧ | |
| D�� | ��pH��ֱֽ�Ӳ�����Һ�в���ij��ҺpH |
| A�� | ������ʲô�����£�c��H+���������Զ���ǿ | |
| B�� | ϡ���Ȼ����Һ��ˮ�ĵ���̶ȱ�� | |
| C�� | ϡ������������Һ��ˮ�ĵ���̶ȱ�С | |
| D�� | �����¶ȣ�KW���H+Ũ������ |
 ��
��