��Ŀ����
ij�ֺ������������ƵĹ���������������֪��������Ϊ
| �������� | ������g�� |
��ƿ+ˮ+���� | ��һ�� | 192.214 |
�ڶ��� | 192.164 | |
������ | 192.028 | |
���Ĵ� | 192.010 | |
����� | 192.010 |
(1)д��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ ��
(2)����������Ƶ���������ʱ������������� �������������ζ�����ԭ���� ��
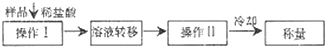
(3)�ⶨ������Ʒ��
![]()
�ٲ������������ ��
����ֱ�Ӳⶨ���������� ��
�۲ⶨ��������Ҫ�������е�����ƽ�������ƾ��ƣ�����Ҫ �� ���̶����г��������⣩��
����ת����Һʱ������Һת�Ʋ���ȫ����Na2O2�����������IJⶨ��� ���ƫ��ƫС�����䡱����
��������1��2Na2O2+2H2O![]() 4NaOH+O2��
4NaOH+O2��
��2��w(Na2O2)= ![]() ��100%������w(Na2O2)�����m(����)��֪���������m(Na2O2)��Ҫ������Ӧ�Ļ�ѧ����ʽ���������������ݼ��������ʵ������е�����ƽ��ʾ�����������ϼ�С�������ɷ�Ӧ������O2�ݳ�������ġ�Na2O2��ȫ��Ӧʱ������O2������������������ƿ��ˮ����������ȥ��Ӧ��ֹʱ��ƿ�ͷ�Ӧ���Һ����������������ƽ���Ĵλ�������ʾ����������������Ӧ�ķ���ʽ�Ͳ���O2���������ɼ�������������m(Na2O2)���ۺ����Ϸ���������Na2O2����������ʱ����Ҫ������������ƿ��ˮ�����������ģ����壩�ζ��������þ�����ֵ��ʾ�������������ݡ������������ζ�����ԭ���ǵ��ĺ͵���ζ���ʱ��ƿ�������Ѵ���ء���3�������м�������ʱ�������з�Ӧ��Na2O2+2HCl
��100%������w(Na2O2)�����m(����)��֪���������m(Na2O2)��Ҫ������Ӧ�Ļ�ѧ����ʽ���������������ݼ��������ʵ������е�����ƽ��ʾ�����������ϼ�С�������ɷ�Ӧ������O2�ݳ�������ġ�Na2O2��ȫ��Ӧʱ������O2������������������ƿ��ˮ����������ȥ��Ӧ��ֹʱ��ƿ�ͷ�Ӧ���Һ����������������ƽ���Ĵλ�������ʾ����������������Ӧ�ķ���ʽ�Ͳ���O2���������ɼ�������������m(Na2O2)���ۺ����Ϸ���������Na2O2����������ʱ����Ҫ������������ƿ��ˮ�����������ģ����壩�ζ��������þ�����ֵ��ʾ�������������ݡ������������ζ�����ԭ���ǵ��ĺ͵���ζ���ʱ��ƿ�������Ѵ���ء���3�������м�������ʱ�������з�Ӧ��Na2O2+2HCl![]() 2NaCl+H2O2��Na2O+2HCl
2NaCl+H2O2��Na2O+2HCl![]() 2NaCl+H2O���Ҳ�����������Ӧ֮���м����ֵ�Ŀɲ����ݣ������ټ���ʹH2O2�ֽ⣬��ʹʣ���HCl��H2O�ӷ��������ܳ���NaCl��������һ�м����ֵ�����ݡ�������һ����˼·������ȷ��ʵ����������еIJ��������ڼ��������·�Ӧ���������Ǽ���������Һ������Ҫֱ�Ӳⶨ�������������ɺ��NaCl���������������Ҫ���������ձ���������������ʱ����Na2O2��Na2O�������ֱ�Ϊm1��m2�������������ݿ��г�������������ʽ��m1+m2=
2NaCl+H2O���Ҳ�����������Ӧ֮���м����ֵ�Ŀɲ����ݣ������ټ���ʹH2O2�ֽ⣬��ʹʣ���HCl��H2O�ӷ��������ܳ���NaCl��������һ�м����ֵ�����ݡ�������һ����˼·������ȷ��ʵ����������еIJ��������ڼ��������·�Ӧ���������Ǽ���������Һ������Ҫֱ�Ӳⶨ�������������ɺ��NaCl���������������Ҫ���������ձ���������������ʱ����Na2O2��Na2O�������ֱ�Ϊm1��m2�������������ݿ��г�������������ʽ��m1+m2=![]() ��2+
��2+![]() ��2)��
��2)��
�𰸣���1��2Na2O2+2H2O![]() 4NaOH+O2��
4NaOH+O2��
��2��������������ƿ��ˮ�����������ģ����壩�ζ��������þ�����ֵ��ʾ�� ��ƿ�������Ѵ����
��3��������
��NaCl������
���ձ�������
��ƫ��

 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�
| �������� | ����/g |
��ƿ+ˮ+���� | ��1�� | 192.214 |
��2�� | 192.164 | |
��3�� | 192.028 | |
��4�� | 192.010 | |
��5�� | 192.010 |
��1��д��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ________________________________��
��2���������������������ʱ�������������________________����������6�ζ�����ԭ����________________________________��
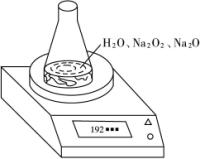
��3���ⶨ������Ʒ��1.560 g����Na2O2������������һ�ַ�����������������£�
��Ʒ��ϡ����
![]()
�ٲ������������____________��
����ֱ�Ӳⶨ����������____________��
�۲ⶨ��������Ҫ�������е�����ƽ�������ƾ��ƣ�����Ҫ________��________���̶����г��������⣩��
����ת����Һʱ������Һת�Ʋ���ȫ����Na2O2���������IJⶨ���____________���ƫ��ƫС�����䡱����
 ij�ֺ������������ƵĹ���������������Ϊ1.56g��Ϊ�ⶨ���ɷֵ���������������ͼ��ʾ��ԭ��ƿ��ˮ��������Ϊ190.72����1.56g������ƷͶ����ƿ�У���ַ�Ӧ������ƽ���յĶ���Ϊ192.12g��
ij�ֺ������������ƵĹ���������������Ϊ1.56g��Ϊ�ⶨ���ɷֵ���������������ͼ��ʾ��ԭ��ƿ��ˮ��������Ϊ190.72����1.56g������ƷͶ����ƿ�У���ַ�Ӧ������ƽ���յĶ���Ϊ192.12g��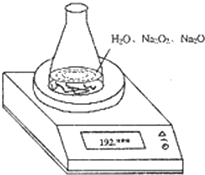 ��2004?�Ϻ���ij�ֺ������������ƵĹ���������������֪��������Ϊ1.560g����ƿ��ˮ������Ϊ190.720g����������ͼװ�òⶨ�������Na2O2������������ÿ����ͬʱ����õ�����ƽ�����������
��2004?�Ϻ���ij�ֺ������������ƵĹ���������������֪��������Ϊ1.560g����ƿ��ˮ������Ϊ190.720g����������ͼװ�òⶨ�������Na2O2������������ÿ����ͬʱ����õ�����ƽ�����������