��Ŀ����
��ѧ��ӦN2+3H2=2NH3�������仯����13ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
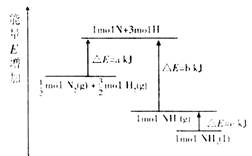

| A��N2(g)+3H2(g)=2NH3(1); ��H=2(a-b-c)kJ��mol-1 |
| B��N2(g)+3H2(g)=2NH3(g); ��H=2(b-a)kJ��mol-1 |
C��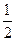 N2(g)+ N2(g)+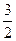 H2(g)=NH3(1); ��H=(b+c-a)kJ��mol-1 H2(g)=NH3(1); ��H=(b+c-a)kJ��mol-1 |
D��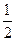 N2(g)+ N2(g)+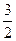 H2(g)=NH3(g); ��H=(a+b)kJ��mol-1 H2(g)=NH3(g); ��H=(a+b)kJ��mol-1 |
A��
��ͼ���Կ�����molN2(g)��molH2(g)������ΪakJ��1molNH3(g)������ΪbkJ������N2(g)��N2(g)===NH3(g)����H��(a��b)kJ/mol����1mol��NH3(g)ת��Ϊ1mol��NH3(l)�ų�������ΪckJ�������У�N2(g)��N2(g)===NH3(l)����H��(a��b��c)kJ/mol������N2(g)+3H2(g)=2NH3(1)��
��H=2(a-b-c)kJ��mol-1��
��H=2(a-b-c)kJ��mol-1��

��ϰ��ϵ�д�
 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ

 C6H12O6��6O2)Ϊ��������Ļ�����ȥ������̼������A���Ը���������ϵ�г�A�ļ���ʽ��
C6H12O6��6O2)Ϊ��������Ļ�����ȥ������̼������A���Ը���������ϵ�г�A�ļ���ʽ��

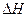 �ľ���ֵ����ȷ��
�ľ���ֵ����ȷ��